Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Diagnosis, Manifestations, & Outcomes I: RA-ILD (0769–0774)
0772: MUC5B Promoter Variant and Survival in Rheumatoid Arthritis-Associated Interstitial Lung Disease
Sunday, November 12, 2023
2:45 PM - 2:55 PM PT
Location: Room 6E/6D
- JK
Jacob Klein, BS
University of Nebraska Medical Center College of Medicine
Omaha, NE, United StatesDisclosure information not submitted.
Presenting Author(s)
Jacob Klein1, Austin Wheeler1, Joshua Baker2, Yangyuna Yang1, Punyasha Roul1, K Wysham3, Gail Kerr4, Andreas Reimold5, Dana Ascherman6, Gary Kunkel7, Grant Cannon8, Paul Monach9, Jill Poole1, Geoffrey Thiele1, Ted R Mikuls10 and Bryant England1, 1University of Nebraska Medical Center, Omaha, NE, 2University of Pennsylvania, Philadelphia, PA, 3VA Puget Sound/University of Washington, Seattle, WA, 4Washington DC VAMC/Georgetown and Howard Universities, Washington, DC, 5University of Texas Southwestern Medical Center, Dallas, TX, 6University of Pittsburgh, Pittsburgh, PA, 7University of Utah, Salt Lake City, UT, 8University of Utah and Salt Lake City VA, Salt Lake City, UT, 9VA Boston Healthcare System, Boston, MA, 10Division of Rheumatology and Immunology, University of Nebraska Medical Center, Omaha, NE
Background/Purpose: The gain of function MUC5B rs35705950 promoter variant is the strongest genetic risk factor for the development of RA-ILD (specific to a usual interstitial pneumonia (UIP) pattern) and idiopathic pulmonary fibrosis (IPF). Since it remains uncertain whether the variant also impacts disease prognosis in RA-ILD or IPF, we examined the association of the MUC5B promoter variant with survival in a multicenter cohort of U.S. Veterans with RA-ILD.
Methods: We studied participants in the Veteran Affairs Rheumatoid Arthritis (VARA) registry with validated ILD diagnoses based on standardized medical record review requiring a provider diagnosis of RA-ILD and either imaging or biopsy findings consistent with ILD. Participants were followed from the latest of either VARA enrollment or ILD diagnosis (index date; to prevent immortal time bias) until death or the end of the study period (12/31/2019). The MUC5B rs35705950 promoter variant was measured via the Infinium Global Screening Array-24 v2.0 (Illumina, Inc; San Diego, CA). The major allele was the guanine (G) nucleotide while the minor allele was the thymine (T) nucleotide. An autosomal dominant inheritance pattern was assumed. Survival was determined from Veterans Affairs death records and linkage with the National Death Index. Kaplan-Meier curves were generated and stratified by the MUC5B variant. Associations of the MUC5B promoter variant with survival were tested in unadjusted and adjusted Cox regression models adjusting for age, sex, smoking history, baseline DAS28, baseline FVC % predicted, and ILD duration. To explore potential survival bias among prevalent ILD cases, a subgroup analysis was completed examining MUC5B status and survival among only incident RA-ILD cases. A sensitivity analysis was also performed restricting to participants with UIP or honeycombing on chest computed tomography (CT) reports.
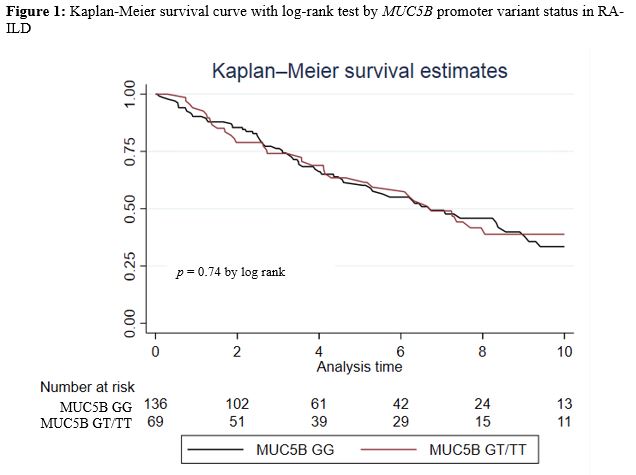
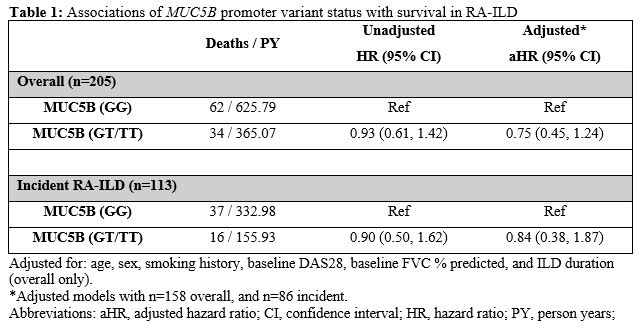
Results: We studied 205 participants with RA-ILD (mean age 69 years, 94% male, 73% white). A smoking history was present in 85%, and the mean ILD duration at index date was 1.7 years. The MUC5B promoter variant was detected in 33.7%. Over 990 patient-years of follow-up, 96 deaths occurred. Mortality rate was similar between those with (9.3/100PY [6.7, 13.0]) and without (9.9/100PY [7.7, 12.7]) the variant (Figure 1; p = 0.74 by log rank test). In the overall RA-ILD cohort, MUC5B status was not significantly associated with survival in unadjusted or adjusted (aHR 0.75 [0.45, 1.24]) models (Table 1). Restricting RA-ILD to incident cases produced similar results, with no significant association between the MUC5B promoter variant and survival in unadjusted or adjusted (aHR 0.84 [0.38, 1.87]) models (Table 1). Findings were similar when restricting to participants with UIP or honeycombing on chest CT (data not shown).
Conclusion: While associated with RA-ILD risk, the MUC5B gain of function promoter variant was not predictive of survival in this multicenter RA-ILD cohort. Further studies are needed to identify and evaluate other potential genetic and non-genetic prognostic factors in RA-ILD to inform disease prognostication and management.
J. Klein: None; A. Wheeler: None; J. Baker: Bristol-Myers Squibb(BMS), 2, Burns-White, LLC, 2, CorEvitas, LLC, 2, Pfizer, 2; Y. Yang: None; P. Roul: None; K. Wysham: None; G. Kerr: AstraZeneca, 2, Aurinia, 6, Horizon, 2, Janssen, 2, Pfizer, 1, Sanofi, 2; A. Reimold: None; D. Ascherman: None; G. Kunkel: None; G. Cannon: None; P. Monach: Genentech, 12, Lecture with honorarium, HI-Bio, 2; J. Poole: AstraZeneca, 12, I have received no monies. I received anti-IL-33 monoclonal antibody from AstraZeneca for animal research sutdies.; G. Thiele: None; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9; B. England: Boehringer-Ingelheim, 2, 5.
Background/Purpose: The gain of function MUC5B rs35705950 promoter variant is the strongest genetic risk factor for the development of RA-ILD (specific to a usual interstitial pneumonia (UIP) pattern) and idiopathic pulmonary fibrosis (IPF). Since it remains uncertain whether the variant also impacts disease prognosis in RA-ILD or IPF, we examined the association of the MUC5B promoter variant with survival in a multicenter cohort of U.S. Veterans with RA-ILD.
Methods: We studied participants in the Veteran Affairs Rheumatoid Arthritis (VARA) registry with validated ILD diagnoses based on standardized medical record review requiring a provider diagnosis of RA-ILD and either imaging or biopsy findings consistent with ILD. Participants were followed from the latest of either VARA enrollment or ILD diagnosis (index date; to prevent immortal time bias) until death or the end of the study period (12/31/2019). The MUC5B rs35705950 promoter variant was measured via the Infinium Global Screening Array-24 v2.0 (Illumina, Inc; San Diego, CA). The major allele was the guanine (G) nucleotide while the minor allele was the thymine (T) nucleotide. An autosomal dominant inheritance pattern was assumed. Survival was determined from Veterans Affairs death records and linkage with the National Death Index. Kaplan-Meier curves were generated and stratified by the MUC5B variant. Associations of the MUC5B promoter variant with survival were tested in unadjusted and adjusted Cox regression models adjusting for age, sex, smoking history, baseline DAS28, baseline FVC % predicted, and ILD duration. To explore potential survival bias among prevalent ILD cases, a subgroup analysis was completed examining MUC5B status and survival among only incident RA-ILD cases. A sensitivity analysis was also performed restricting to participants with UIP or honeycombing on chest computed tomography (CT) reports.
Results: We studied 205 participants with RA-ILD (mean age 69 years, 94% male, 73% white). A smoking history was present in 85%, and the mean ILD duration at index date was 1.7 years. The MUC5B promoter variant was detected in 33.7%. Over 990 patient-years of follow-up, 96 deaths occurred. Mortality rate was similar between those with (9.3/100PY [6.7, 13.0]) and without (9.9/100PY [7.7, 12.7]) the variant (Figure 1; p = 0.74 by log rank test). In the overall RA-ILD cohort, MUC5B status was not significantly associated with survival in unadjusted or adjusted (aHR 0.75 [0.45, 1.24]) models (Table 1). Restricting RA-ILD to incident cases produced similar results, with no significant association between the MUC5B promoter variant and survival in unadjusted or adjusted (aHR 0.84 [0.38, 1.87]) models (Table 1). Findings were similar when restricting to participants with UIP or honeycombing on chest CT (data not shown).
Conclusion: While associated with RA-ILD risk, the MUC5B gain of function promoter variant was not predictive of survival in this multicenter RA-ILD cohort. Further studies are needed to identify and evaluate other potential genetic and non-genetic prognostic factors in RA-ILD to inform disease prognostication and management.

Figure 1. Kaplan-Meier survival curve with log-rank test by MUC5B promoter variant status in RA-ILD.

Table 1. Associations of MUC5B promoter variant status with survival in RA-ILD
J. Klein: None; A. Wheeler: None; J. Baker: Bristol-Myers Squibb(BMS), 2, Burns-White, LLC, 2, CorEvitas, LLC, 2, Pfizer, 2; Y. Yang: None; P. Roul: None; K. Wysham: None; G. Kerr: AstraZeneca, 2, Aurinia, 6, Horizon, 2, Janssen, 2, Pfizer, 1, Sanofi, 2; A. Reimold: None; D. Ascherman: None; G. Kunkel: None; G. Cannon: None; P. Monach: Genentech, 12, Lecture with honorarium, HI-Bio, 2; J. Poole: AstraZeneca, 12, I have received no monies. I received anti-IL-33 monoclonal antibody from AstraZeneca for animal research sutdies.; G. Thiele: None; T. Mikuls: Elsevier, 9, Horizon Therapeutics, 2, 5, Pfizer, 2, Sanofi, 2, UCB Pharma, 2, Wolters Kluwer Health (UpToDate), 9; B. England: Boehringer-Ingelheim, 2, 5.



