Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III: SpA
2230: Bimekizumab Treatment in Patients with Active PsA and Prior Inadequate Response to TNF Inhibitors: Sustained Efficacy and Safety Results from a Phase 3 Study and Its Open-Label Extension up to 1 Year
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Laura Coates, MD, PhD
University of Oxford
Oxford, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Laura Coates1, Robert BM Landewé2, Iain McInnes3, Philip J. Mease4, Christopher T Ritchlin5, Yoshiya Tanaka6, Akihiko Asahina7, Frank Behrens8, Dafna Gladman9, Laure Gossec10, Alice B. Gottlieb11, Richard B. Warren12, Barbara Ink13, Rajan Bajracharya13, Jason Coarse14 and Joseph Merola15, 1University of Oxford, Oxford, United Kingdom, 2Amsterdam Rheumatology & Clinical Immunology Center, Amsterdam and Zuyderland MC, Herleen, Netherlands, 3University of Glasgow, Glasgow, United Kingdom, 4Swedish Medical Center/Providence St. Joseph Health and University of Washington School of Medicine, Seattle, WA, 5University of Rochester Medical School, Allergy, Immunology & Rheumatology Division, Canandaigua, NY, 6University of Occupational and Environmental Health, Kitakyushu, Japan, 7The Jikei University School of Medicine, Department of Dermatology, Tokyo, Japan, 8Goethe University, Division of Rheumatology, University Hospital and Fraunhofer Institute for Translational Medicine & Pharmacology, Frankfurt, Germany, 9Schroeder Arthritis Institute, Krembil Research Institute, Toronto Western Hospital, Department of Medicine, University of Toronto, Toronto, ON, Canada, 10Sorbonne Université and Pitié Salpêtrière Hospital, Paris, France, 11Icahn School of Medicine at Mount Sinai, New York, NY, 12Dermatology Centre, Northern Care Alliance NHS Foundation Trust; NIHR Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, United Kingdom, 13UCB Pharma, Slough, United Kingdom, 14UCB Pharma, Morrisville, NC, 15Harvard Medical School, Brigham and Women's Hospital, Newton, MA
Background/Purpose: Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown superior efficacy to 16 weeks (wks) vs placebo (PBO) and tolerability in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.1,2 Efficacy of BKZ to 52 wks has also been demonstrated in the BE OPTIMAL study in biologic-naïve pts with PsA.3 Here, the efficacy and safety of BKZ treatment in pts with active PsA and prior inadequate response or intolerance to TNF inhibitors (TNFi-IR) are reported up to Wk 52 in the BE COMPLETE study.
Methods: BE COMPLETE (NCT03896581) included a 16-wk double-blind, PBO-controlled period. Wk 16 completers were eligible for entry into BE VITAL (NCT04009499; open-label extension). Pts were randomized 2:1 to subcutaneous BKZ 160 mg every 4 wks or PBO. At Wk 16, PBO pts switched to BKZ (PBO/BKZ; received 36 wks of BKZ treatment up to Wk 52). BE VITAL included pts from BE OPTIMAL and BE COMPLETE; data here are for pts randomized at baseline (BL [Wk 0]) of BE COMPLETE only, up to 52 wks. Efficacy data are reported as observed case (OC) or using non‑responder imputation (NRI; binary) or multiple imputation (MI; continuous). The number of treatment-emergent adverse events (TEAEs) to Wk 52 are reported for pts who received ≥1 dose of BKZ.
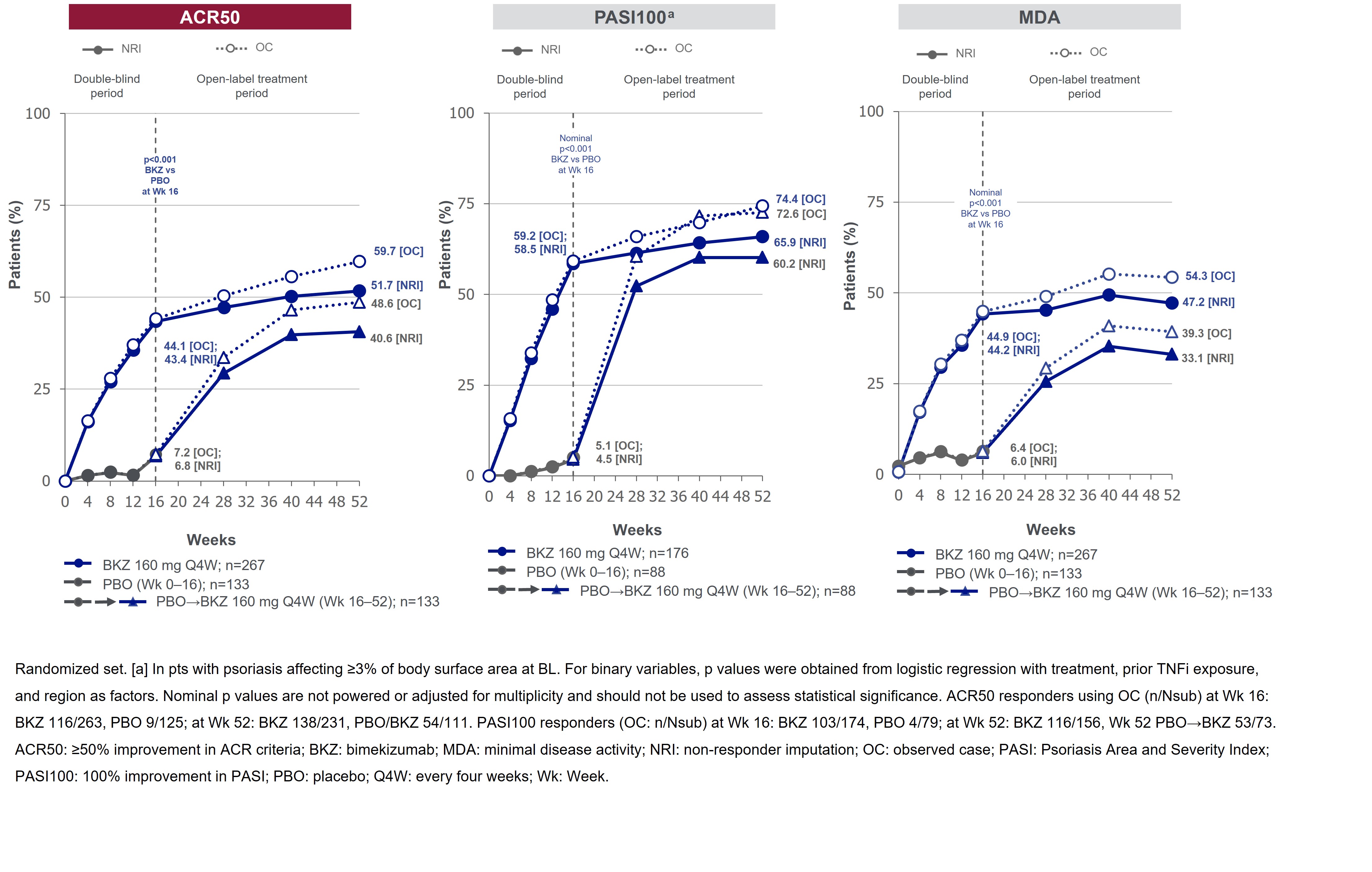
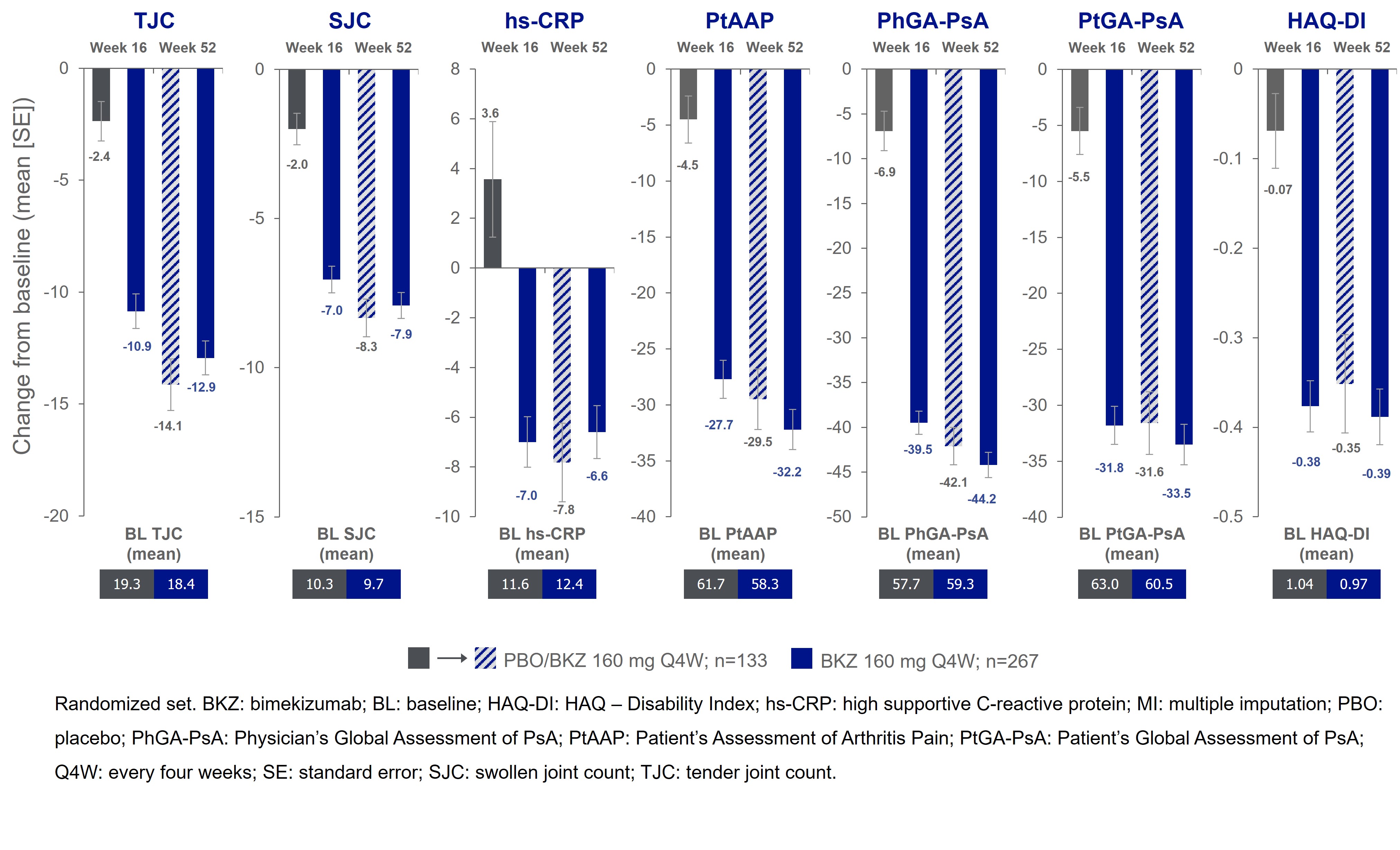
Results: 388/400 (97.0%) pts completed Wk 16; 377 (94.3%) entered BE VITAL and 347 (86.8%) completed Wk 52. Improved joint and skin efficacy responses with BKZ treatment were sustained from Wk 16 to Wk 52 (Table). At Wk 52, 138/267 (51.7%) of BKZ and 54/133 (40.6%) of PBO/BKZ pts achieved ACR50 (Figure 1). Improvements from BL in all ACR components were seen at Wk 16 and sustained to Wk 52 in BKZ-treated pts (Figure 2). In pts with BL psoriasis (≥3% body surface area), 116/176 (65.9%) of BKZ and 53/88 (60.2%) of PBO/BKZ pts achieved complete skin clearance (Psoriasis Area Severity Index [PASI]100) at Wk 52. At Wk 52, 126/267 (47.2%) of BKZ and 44/133 (33.1%) of PBO/BKZ pts achieved minimal disease activity (MDA; Figure 1). To Wk 52, 243/388 (62.6%) pts had ≥1 TEAE whilst receiving BKZ (exposure-adjusted incidence rate per 100 pt-years [EAIR/100 PY]: 126.0); 23 (5.9%) pts reported a serious TEAE (7.0/100 PY). Malignancies (excluding nonmelanoma skin cancers) were reported by 2 (0.7%) pts receiving BKZ (0.77/100 PY). Candida infections were reported by 25 (6.4%) pts receiving BKZ (7.7/100 PY); all were reported as mild or moderate by investigators and none were systemic. Two cases of oral candidiasis led to study discontinuation. There was one death (sudden death; pt with history of cardiac events), two adjudicated major adverse cardiac events and no definite or probable adjudicated inflammatory bowel disease.
Conclusion: In pts with PsA and TNFi-IR, BKZ demonstrated sustained clinical efficacy from Wk 16 up to Wk 52. The safety profile was consistent with previous reports.1–3
References: 1. McInnes IB. Lancet 2023; 401(25–37); 2. Merola JF. Lancet 2023; 401(38–48); 3. Ritchlin C. Arthritis Rheumatol 2022;74(S9).
.jpg)
L. Coates: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Biogen, 6, Bristol Myers Squibb, 2, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Galapagos, 2, 6, Gilead Sciences, 2, 6, GSK, 6, Janssen, 2, 5, 6, Medac, 6, MoonLake, 2, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5, 6; R. Landewé: AbbVie, 2, 5, AstraZeneca, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, 5, Pfizer, 2, 5, Rheumatology Consultancy BV, 12, Owner, UCB Pharma, 2, 5; I. McInnes: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, 5, Cabaletta, 2, 11, Causeway Therapeutics, 2, 11, Celgene, 2, 5, Compugen, 2, 11, Dextera, 11, Eli Lilly, 2, EveloBio, 1, 2, 4, 11, Gilead, 2, Janssen, 2, 5, Moonlake, 2, NHS GGC, 4, Novartis, 2, 5, Pfizer, 2, Sanofi, 2, UCB, 2, 5, Versus Arthritis, 12, Trustee Status; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, Aclaris, 2, Amgen, 2, 5, 6, Boehringer Ingelheim, 2, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, 6, Galapagos, 2, Gilead, 2, GlaxoSmithKline, 2, Inmagene, 2, Janssen, 2, 5, 6, MoonLake Pharma, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sun Pharma, 2, 5, UCB Pharma, 2, 5, 6, Ventyx, 2, Xinthera, 2; C. Ritchlin: AbbVie, 2, 5, 6, Amgen, 2, BMS, 2, Eli Lilly, 2, Gilead, 2, Janssen, 2, Novartis, 2, Pfizer, 2, 5, 6, UCB, 2, 6; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; A. Asahina: AbbVie, 5, Amgen, 5, BMS, 5, Boehringer Ingelheim, 5, Eisai, 5, Eli Lilly, 5, Kyowa Kirin, 5, LEO Pharma, 5, Maruho, 5, Mitsubishi Tanabe Pharma, 5, Pfizer, 5, Sun Pharma, 5, Taiho Pharma, 5, Torii Pharmaceutical Co., 5, UCB Pharma, 5; F. Behrens: AbbVie, 2, 6, Affibody, 2, Amgen, 6, Boehringer-Ingelheim, 2, Celgene, 5, Chugai, 5, Eli Lilly, 6, Genzyme, 6, Gilead Sciences, 2, GSK, 2, 6, Janssen, 2, 5, MoonLake, 2, 6, MSD, 2, 6, Novartis, 6, Pfizer, 2, 5, 6, Roche, 5, Sandoz, 2, 6, Sanofi, 2, 6; D. Gladman: AbbVie, 2, 5, Amgen, 2, 5, Bristol Myers Squibb, 2, Celgene, 2, 5, Eli Lilly, 2, 5, Galapagos, 2, Gilead Sciences, 2, Janssen, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, UCB, 2, 5; L. Gossec: AbbVie, 2, 12, Personal fees, Amgen, 2, Biogen, 5, BMS, 12, Personal fees, Celltrion, 12, Personal fees, Eli Lilly, 5, 12, Personal fees, Galapagos, 12, Personal fees, Janssen, 12, Personal fees, MSD, 12, Personal fees, Novartis, 5, 12, Personal fees, Pfizer, 12, Personal fees, Sandoz, 5, 12, Personal fees, UCB Pharma, 5, 12, Personal fees; A. Gottlieb: Amgen, 1, 2, AnaptysBio, 1, 2, 5, Avotres Therapeutics, 1, 2, Boehringer Ingelheim, 1, 2, Bristol Myers Squibb, 1, 2, 5, Dice Therapeutics, 1, 2, Eli Lilly, 1, 2, Janssen, 1, 2, MoonLake Immunotherapeutics, 5, Novartis, 1, 2, 5, Sanofi, 1, 2, UCB Pharma, 1, 2, 5, XBiotech, 1, 2; R. Warren: AbbVie, 2, 5, 6, Almirall, 2, 5, 6, Amgen, 2, 5, 6, Arena, 2, 6, Astellas, 2, 6, Avillion, 2, 6, Biogen, 2, 6, BMS, 2, 6, Boehringer Ingelheim, 2, 6, Celgene, 2, 5, DiCE, 6, Eli Lilly, 2, 5, 6, GSK, 2, 6, Janssen, 2, 5, 6, LEO Pharma, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sanofi, 2, 6, Sun Pharma, 6, UCB Pharma, 2, 5, 6, Union, 6; B. Ink: AbbVie, 11, GSK, 11, UCB Pharma, 3, 11; R. Bajracharya: UCB Pharma, 3, 11; J. Coarse: UCB Pharma, 3, 11; J. Merola: Abbvie, 2, 12, Investigator, Amgen, 2, 12, Investigator, Biogen, 2, 12, Investigator, Bristol-Myers Squibb, 2, 12, Investigator, Dermavant, 2, 12, Investigator, Eli Lilly, 2, 6, 12, Investigator, Janssen, 2, 12, Investigator, Leo Pharma, 2, 12, Investigator, Novartis, 2, 12, Investigator, Pfizer, 2, 12, Investigator, Regeneron, 2, 12, Investigator, Sanofi, 2, 12, Investigator, Sun Pharma, 2, 12, Investigator, UCB Pharma, 2, 12, Investigator.
Background/Purpose: Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, has shown superior efficacy to 16 weeks (wks) vs placebo (PBO) and tolerability in patients (pts) with active PsA in the phase 3 BE OPTIMAL and BE COMPLETE studies.1,2 Efficacy of BKZ to 52 wks has also been demonstrated in the BE OPTIMAL study in biologic-naïve pts with PsA.3 Here, the efficacy and safety of BKZ treatment in pts with active PsA and prior inadequate response or intolerance to TNF inhibitors (TNFi-IR) are reported up to Wk 52 in the BE COMPLETE study.
Methods: BE COMPLETE (NCT03896581) included a 16-wk double-blind, PBO-controlled period. Wk 16 completers were eligible for entry into BE VITAL (NCT04009499; open-label extension). Pts were randomized 2:1 to subcutaneous BKZ 160 mg every 4 wks or PBO. At Wk 16, PBO pts switched to BKZ (PBO/BKZ; received 36 wks of BKZ treatment up to Wk 52). BE VITAL included pts from BE OPTIMAL and BE COMPLETE; data here are for pts randomized at baseline (BL [Wk 0]) of BE COMPLETE only, up to 52 wks. Efficacy data are reported as observed case (OC) or using non‑responder imputation (NRI; binary) or multiple imputation (MI; continuous). The number of treatment-emergent adverse events (TEAEs) to Wk 52 are reported for pts who received ≥1 dose of BKZ.
Results: 388/400 (97.0%) pts completed Wk 16; 377 (94.3%) entered BE VITAL and 347 (86.8%) completed Wk 52. Improved joint and skin efficacy responses with BKZ treatment were sustained from Wk 16 to Wk 52 (Table). At Wk 52, 138/267 (51.7%) of BKZ and 54/133 (40.6%) of PBO/BKZ pts achieved ACR50 (Figure 1). Improvements from BL in all ACR components were seen at Wk 16 and sustained to Wk 52 in BKZ-treated pts (Figure 2). In pts with BL psoriasis (≥3% body surface area), 116/176 (65.9%) of BKZ and 53/88 (60.2%) of PBO/BKZ pts achieved complete skin clearance (Psoriasis Area Severity Index [PASI]100) at Wk 52. At Wk 52, 126/267 (47.2%) of BKZ and 44/133 (33.1%) of PBO/BKZ pts achieved minimal disease activity (MDA; Figure 1). To Wk 52, 243/388 (62.6%) pts had ≥1 TEAE whilst receiving BKZ (exposure-adjusted incidence rate per 100 pt-years [EAIR/100 PY]: 126.0); 23 (5.9%) pts reported a serious TEAE (7.0/100 PY). Malignancies (excluding nonmelanoma skin cancers) were reported by 2 (0.7%) pts receiving BKZ (0.77/100 PY). Candida infections were reported by 25 (6.4%) pts receiving BKZ (7.7/100 PY); all were reported as mild or moderate by investigators and none were systemic. Two cases of oral candidiasis led to study discontinuation. There was one death (sudden death; pt with history of cardiac events), two adjudicated major adverse cardiac events and no definite or probable adjudicated inflammatory bowel disease.
Conclusion: In pts with PsA and TNFi-IR, BKZ demonstrated sustained clinical efficacy from Wk 16 up to Wk 52. The safety profile was consistent with previous reports.1–3
References: 1. McInnes IB. Lancet 2023; 401(25–37); 2. Merola JF. Lancet 2023; 401(38–48); 3. Ritchlin C. Arthritis Rheumatol 2022;74(S9).
.jpg)
Table. BKZ efficacy at Weeks 16 and 52 [NRI and MI]

Figure 1. ACR50, PASI100 and MDA responses over time up to Week 52 [NRI and OC]

Figure 2. Change from BL in the ACR components to 52 weeks [MI]
L. Coates: AbbVie, 2, 5, 6, Amgen, 2, 5, 6, Biogen, 6, Bristol Myers Squibb, 2, Celgene, 2, 5, 6, Eli Lilly, 2, 5, 6, Galapagos, 2, 6, Gilead Sciences, 2, 6, GSK, 6, Janssen, 2, 5, 6, Medac, 6, MoonLake, 2, Novartis, 2, 5, 6, Pfizer Inc, 2, 5, 6, UCB, 2, 5, 6; R. Landewé: AbbVie, 2, 5, AstraZeneca, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, 5, Pfizer, 2, 5, Rheumatology Consultancy BV, 12, Owner, UCB Pharma, 2, 5; I. McInnes: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, 5, Cabaletta, 2, 11, Causeway Therapeutics, 2, 11, Celgene, 2, 5, Compugen, 2, 11, Dextera, 11, Eli Lilly, 2, EveloBio, 1, 2, 4, 11, Gilead, 2, Janssen, 2, 5, Moonlake, 2, NHS GGC, 4, Novartis, 2, 5, Pfizer, 2, Sanofi, 2, UCB, 2, 5, Versus Arthritis, 12, Trustee Status; P. Mease: AbbVie, 2, 5, 6, Acelyrin, 2, Aclaris, 2, Amgen, 2, 5, 6, Boehringer Ingelheim, 2, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, 6, Galapagos, 2, Gilead, 2, GlaxoSmithKline, 2, Inmagene, 2, Janssen, 2, 5, 6, MoonLake Pharma, 2, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sun Pharma, 2, 5, UCB Pharma, 2, 5, 6, Ventyx, 2, Xinthera, 2; C. Ritchlin: AbbVie, 2, 5, 6, Amgen, 2, BMS, 2, Eli Lilly, 2, Gilead, 2, Janssen, 2, Novartis, 2, Pfizer, 2, 5, 6, UCB, 2, 6; Y. Tanaka: AbbVie, 6, AstraZeneca, 6, BMS, 6, Boehringer-Ingelheim, 6, Chugai, 5, 6, Eisai, 5, 6, Eli Lilly, 6, Gilead, 6, GSK, 6, Mitsubishi-Tanabe, 5, Pfizer, 6, Taiho, 6, Taisho, 5, 6; A. Asahina: AbbVie, 5, Amgen, 5, BMS, 5, Boehringer Ingelheim, 5, Eisai, 5, Eli Lilly, 5, Kyowa Kirin, 5, LEO Pharma, 5, Maruho, 5, Mitsubishi Tanabe Pharma, 5, Pfizer, 5, Sun Pharma, 5, Taiho Pharma, 5, Torii Pharmaceutical Co., 5, UCB Pharma, 5; F. Behrens: AbbVie, 2, 6, Affibody, 2, Amgen, 6, Boehringer-Ingelheim, 2, Celgene, 5, Chugai, 5, Eli Lilly, 6, Genzyme, 6, Gilead Sciences, 2, GSK, 2, 6, Janssen, 2, 5, MoonLake, 2, 6, MSD, 2, 6, Novartis, 6, Pfizer, 2, 5, 6, Roche, 5, Sandoz, 2, 6, Sanofi, 2, 6; D. Gladman: AbbVie, 2, 5, Amgen, 2, 5, Bristol Myers Squibb, 2, Celgene, 2, 5, Eli Lilly, 2, 5, Galapagos, 2, Gilead Sciences, 2, Janssen, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, UCB, 2, 5; L. Gossec: AbbVie, 2, 12, Personal fees, Amgen, 2, Biogen, 5, BMS, 12, Personal fees, Celltrion, 12, Personal fees, Eli Lilly, 5, 12, Personal fees, Galapagos, 12, Personal fees, Janssen, 12, Personal fees, MSD, 12, Personal fees, Novartis, 5, 12, Personal fees, Pfizer, 12, Personal fees, Sandoz, 5, 12, Personal fees, UCB Pharma, 5, 12, Personal fees; A. Gottlieb: Amgen, 1, 2, AnaptysBio, 1, 2, 5, Avotres Therapeutics, 1, 2, Boehringer Ingelheim, 1, 2, Bristol Myers Squibb, 1, 2, 5, Dice Therapeutics, 1, 2, Eli Lilly, 1, 2, Janssen, 1, 2, MoonLake Immunotherapeutics, 5, Novartis, 1, 2, 5, Sanofi, 1, 2, UCB Pharma, 1, 2, 5, XBiotech, 1, 2; R. Warren: AbbVie, 2, 5, 6, Almirall, 2, 5, 6, Amgen, 2, 5, 6, Arena, 2, 6, Astellas, 2, 6, Avillion, 2, 6, Biogen, 2, 6, BMS, 2, 6, Boehringer Ingelheim, 2, 6, Celgene, 2, 5, DiCE, 6, Eli Lilly, 2, 5, 6, GSK, 2, 6, Janssen, 2, 5, 6, LEO Pharma, 2, 5, 6, Novartis, 2, 5, 6, Pfizer, 2, 5, 6, Sanofi, 2, 6, Sun Pharma, 6, UCB Pharma, 2, 5, 6, Union, 6; B. Ink: AbbVie, 11, GSK, 11, UCB Pharma, 3, 11; R. Bajracharya: UCB Pharma, 3, 11; J. Coarse: UCB Pharma, 3, 11; J. Merola: Abbvie, 2, 12, Investigator, Amgen, 2, 12, Investigator, Biogen, 2, 12, Investigator, Bristol-Myers Squibb, 2, 12, Investigator, Dermavant, 2, 12, Investigator, Eli Lilly, 2, 6, 12, Investigator, Janssen, 2, 12, Investigator, Leo Pharma, 2, 12, Investigator, Novartis, 2, 12, Investigator, Pfizer, 2, 12, Investigator, Regeneron, 2, 12, Investigator, Sanofi, 2, 12, Investigator, Sun Pharma, 2, 12, Investigator, UCB Pharma, 2, 12, Investigator.



