Poster Session C
Metabolic bone disease
Session: (1996–2018) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
2015: Synergistic Effect Between Denosumab and Immune Checkpoint Inhibitors : A Retrospective Study of 268 Patients with Bone Metastases
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- EM
Abstract Poster Presenter(s)
Emmanuel Massy1, Etienne Mabrut1, Sabine Mainbourg2 and Cyrille Confavreux1, 1Centre Expert des Métastases Osseuses (CEMOS) - Service de Rhumatologie, Centre Hospitalier Lyon-Sud, Hospices Civils de Lyon, Université de Lyon, INSERM UMR 1033-LYOS, Lyon, France, 2Service de médecine interne et vasculaire, Centre Hospitalier Lyon Sud, Hospices Civils de Lyon, F-69495 Pierre-Bénite, Laboratoire de Biométrie et Biologie Evolutive, Université Lyon 1; CNRS, UMR 5558, 69622 Villeurbanne, Lyon, France
Background/Purpose: Bone is the third site of metastasis. Bone metastases are associated with poorer survival of patients and impaired quality of life with the occurrence of skeletal related events. Denosumab (D-MAB), a recombinant fully monoclonal human IgG2 antibody directed against the receptor activator of nuclear factor kappa-B ligand (RANKL) is used for the prevention of skeletal related events (SRE) in advanced solid tumors with bone metastases. RANK/RANKL axis seems also essential in numerous immunological processes. Moreover, oncology had evolved last years with increasing use of Immune Checkpoint Inhibitors (ICI) that suppress tumor-induced immune system inhibition mechanisms. We hypothetized that there could be a synergistic anti-tumor effect between immunotherapy and denosumab according to case-reports, small cohorts and in vivo studies.
Methods: We used a retrospective database in oncology named IMMUCARE and developed in Hospices Civils de Lyon at Centre Hospitalier Lyon Sud. All patients treated with ICI from 2014 are included in this database. We analysed only patients with bone metastases and collected data in their medical file.
We analyzed overall survival (OS), progression-free survival (PFS) and switch of treatment line in different populations according to whether or not use of Denosumab. We designed three different groups, without Denosumab, ICI then Denosumab and Denosumab then ICI. We performed survival curves and Cox model for multivariate analysis.
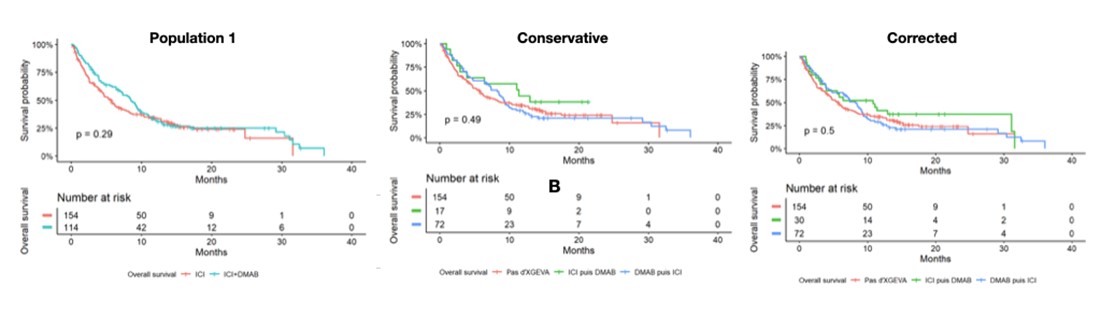
Results: 268 patients presented with bone metastases in the whole cohort. We did not found significant difference for overall survive (OS) and progression free survival (PFS) in favor of combination of Denosumab and ICI but we have a visual impression of superiority on the survival curves in the ICI then D-MAB group. This visual impression of a beneficial effect starting around the 6th month. We identified significant difference for changing of treatment line in the ICI then D-MAB group (p = 0.022) in the conservative population, and at the limit in the corrected population (p = 0.057).
Conclusion: This retrospective study found no statistical benefit of association of D-MAB with ICI in the whole population but a sequence using ICI and then D-MAB seems to be beneficial to patients. It would be interesting to conduct dedicated studies in bigger cohort to confirm these latter statement.
E. Massy: None; E. Mabrut: None; S. Mainbourg: None; C. Confavreux: None.
Background/Purpose: Bone is the third site of metastasis. Bone metastases are associated with poorer survival of patients and impaired quality of life with the occurrence of skeletal related events. Denosumab (D-MAB), a recombinant fully monoclonal human IgG2 antibody directed against the receptor activator of nuclear factor kappa-B ligand (RANKL) is used for the prevention of skeletal related events (SRE) in advanced solid tumors with bone metastases. RANK/RANKL axis seems also essential in numerous immunological processes. Moreover, oncology had evolved last years with increasing use of Immune Checkpoint Inhibitors (ICI) that suppress tumor-induced immune system inhibition mechanisms. We hypothetized that there could be a synergistic anti-tumor effect between immunotherapy and denosumab according to case-reports, small cohorts and in vivo studies.
Methods: We used a retrospective database in oncology named IMMUCARE and developed in Hospices Civils de Lyon at Centre Hospitalier Lyon Sud. All patients treated with ICI from 2014 are included in this database. We analysed only patients with bone metastases and collected data in their medical file.
We analyzed overall survival (OS), progression-free survival (PFS) and switch of treatment line in different populations according to whether or not use of Denosumab. We designed three different groups, without Denosumab, ICI then Denosumab and Denosumab then ICI. We performed survival curves and Cox model for multivariate analysis.
Results: 268 patients presented with bone metastases in the whole cohort. We did not found significant difference for overall survive (OS) and progression free survival (PFS) in favor of combination of Denosumab and ICI but we have a visual impression of superiority on the survival curves in the ICI then D-MAB group. This visual impression of a beneficial effect starting around the 6th month. We identified significant difference for changing of treatment line in the ICI then D-MAB group (p = 0.022) in the conservative population, and at the limit in the corrected population (p = 0.057).
Conclusion: This retrospective study found no statistical benefit of association of D-MAB with ICI in the whole population but a sequence using ICI and then D-MAB seems to be beneficial to patients. It would be interesting to conduct dedicated studies in bigger cohort to confirm these latter statement.

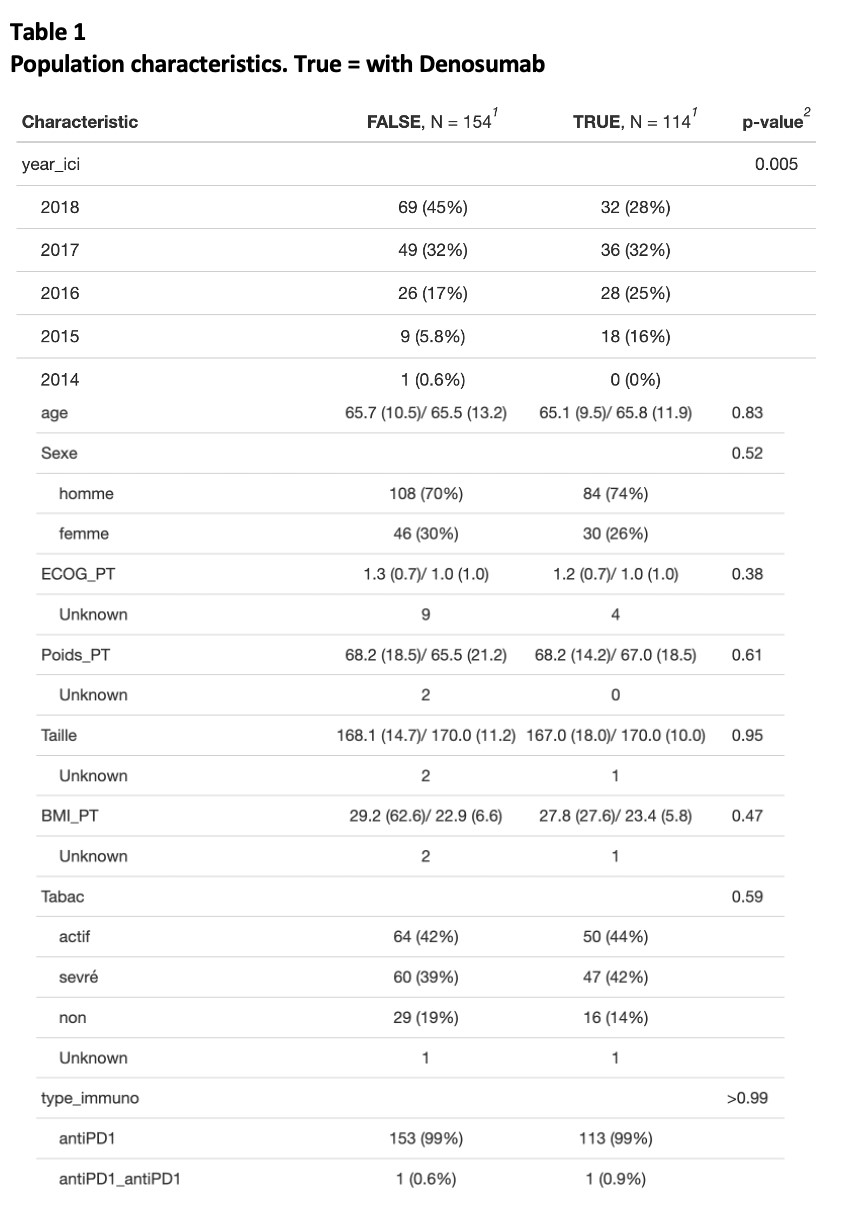
Table 1 - Population characteristics - True = with Denosumab

Figure 1 - Survival curves for overall survival (OS)
E. Massy: None; E. Mabrut: None; S. Mainbourg: None; C. Confavreux: None.



