Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
2294: Organ Damage Progression in Systemic Lupus Erythematosus: An Analysis of the SLE Prospective Observational Cohort Study (SPOCS)
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Richard Furie, MD
Chief of the Division of Rheumatology
Northwell Health
Manhasset, NY, NY, United StatesDisclosure(s): Biogen: Consultant (Ongoing), Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Richard Furie1, Eric Morand2, Christine Peschken3, Martin Aringer4, Laurent Arnaud5, Barnabas Desta6, Tina Grünfeld Eén7, Alessandro Sorrentino8, Stephanie Chen9 and Bo Ding7, 1Northwell Health, Manhasset, NY, 2Monash University, Centre for Inflammatory Diseases, Melbourne, Australia, 3University of Manitoba, Winnipeg, MB, Canada, 4Carl Gustav Carus, Dresden, Germany, 5University Hospitals of Strasbourg, Strasbourg, France, 6BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, MD, 7BioPharmaceuticals Medical, AstraZeneca, Gothenburg, Sweden, 8Medical Affairs, AstraZeneca, Cambridge, United Kingdom, 9BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD
Background/Purpose: The SLE Prospective Observational Cohort Study (SPOCS) is an international, multicenter study collecting data on patients with SLE, including in relation to oral corticosteroid (OCS) use and type 1 interferon gene signature (IFNGS) status. Here, we report SLICC/ACR Damage Index (DI) progression over 3 years in the overall population, as well as by baseline IFNGS category and average daily OCS use.
Methods: Eligible patients (aged ≥18 years) had physician-confirmed moderate-to-severe SLE (ACR or SLICC classification), a ≥6-month treatment duration with systemic SLE treatment beyond non-steroidal anti-inflammatory drugs and analgesics, and ≥1 lifetime positive serology of ANA or dsDNA. Disease organ damage burden was assessed using the SLICC/ACR DI score. IFNGS tests were performed in a central laboratory. The chi-square test was used to compare score categories and the proportions of patients with ≥1 new damage observation since the prior visit; the Kruskal–Wallis test was used for comparisons of the mean (standard deviation [SD]).
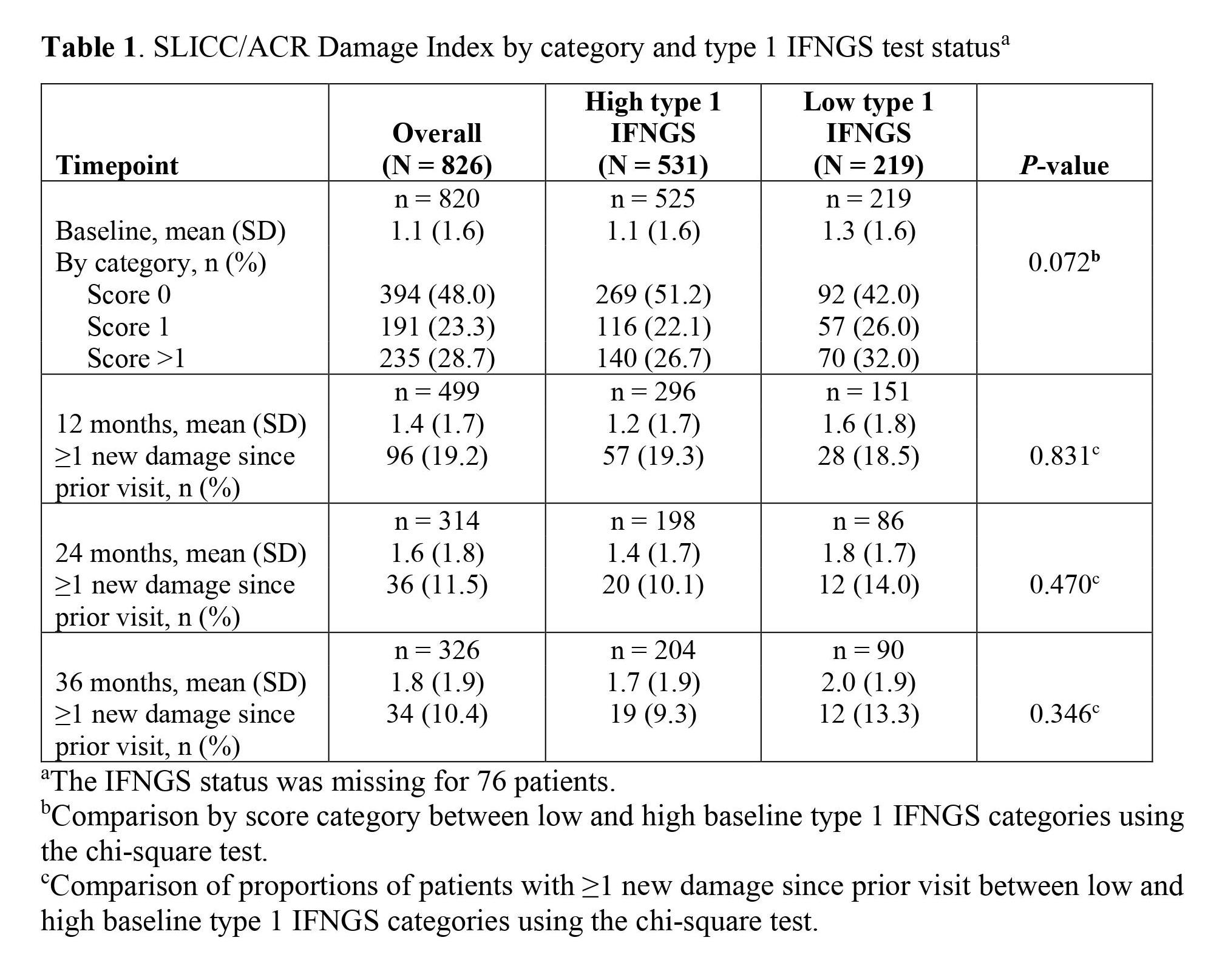
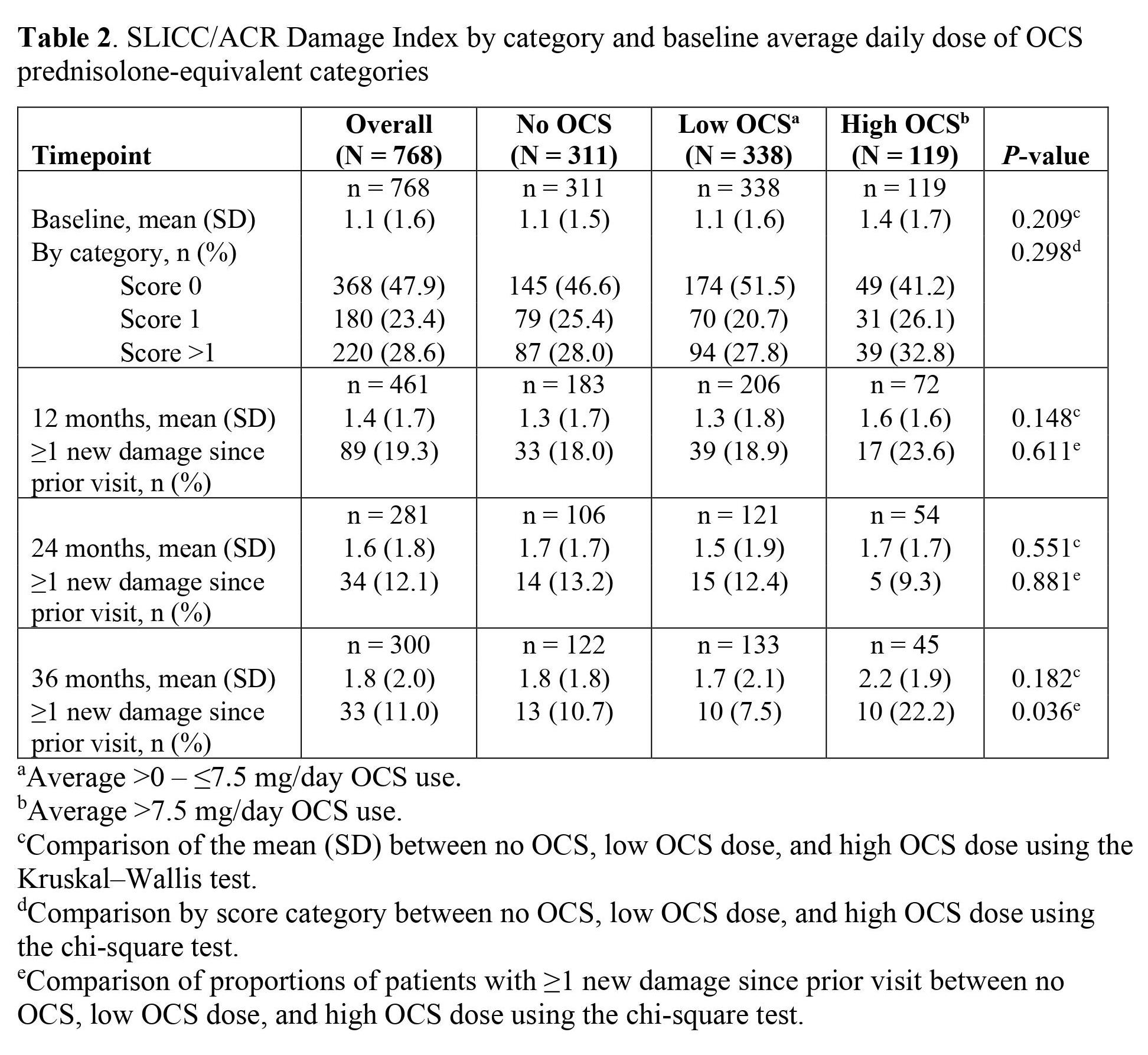
Results: Among 826 patients overall, 70.8% (n = 531) and 29.2% (n = 219) of patients had high and low IFNGS statuses, respectively; IFNGS status was missing for 76 patients. At baseline, 52.0% (n = 426) of patients grouped by high or low IFNGS status and 52.1% (n = 400) of patients grouped by OCS use had organ damage (DI score ≥1). The mean (SD) SLICC/ACR DI score was higher in patients with a low IFNGS than those with a high IFNGS; this trend continued throughout follow-up (Table 1). Average DI scores and percentages of patients with ≥1 new organ damage increased from baseline to 36 months in each category. Among patients stratified by daily OCS use (n = 768), 40.5% (n = 311), 44.0% (n = 338), and 15.5% (n = 119) of patients had no OCS use, low OCS use (0 – ≤7.5 mg/day), and high OCS use ( >7.5 mg/day) at baseline, respectively. Baseline mean (SD) DI scores were also similar in the overall, no OCS use, and low OCS use categories and higher in the high OCS use category (P = 0.209 for the no OCS use, low OCS use, and high OCS use categories; Table 2). The proportion of patients with ≥1 new organ damage increased from baseline to 12 months in each category (P = 0.611 for no OCS use, low OCS use, and high OCS use); a greater percentage of patients had ≥1 new organ damage in the high OCS category compared to the no or low OCS categories. Moreover, the mean (SD) DI score was higher in the high OCS category compared to the no OCS and low OCS categories after 12 months.
Conclusion: Results from the SPOCS study will enhance our understanding of the progression of SLE organ damage and will help improve clinical outcomes for patients with moderate-to-severe SLE. Nearly 20% of patients had new organ damage after 12 months. Patients with low IFNGS had greater organ damage than those with high IFNGS, and compared to no OCS or low daily average OCS use, organ damage is higher in patients with high average daily OCS use. Together, these indicate an unmet need for new treatment options for all patients with SLE, especially steroid-sparing treatments.
R. Furie: Biogen, 2, 5; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; C. Peschken: AstraZeneca, 2, 5, GSK, 2, 5, Roche, 1, 2; M. Aringer: AbbVie/Abbott, 1, 6, Boehringer-Ingelheim, 1, 6, Bristol-Myers Squibb(BMS), 1, 6, Chugai Pharma, 6, Eli Lilly, 1, 6, GlaxoSmithKlein(GSK), 1, 6, Merck/MSD, 6, Novartis, 1, Otsuka Pharma, 1, 6, Pfizer, 1, 6, Roche, 1, 6, Sanofi Aventis, 1, 6, UCB, 1, 6; L. Arnaud: AbbVie, 6, Alexion, 6, Alpine, 2, 6, Amgen, 6, AstraZeneca, 1, 2, 6, Biogen, 6, Boehringer Ingelheim, 6, Bristol-Myers Squibb(BMS), 2, 6, Eli Lilly, 6, GlaxoSmithKlein(GSK), 1, 2, 6, Grifols, 6, Janssen, 6, Kezar Life Sciences, 2, 6, LFB, 6, Medac, 6, Novartis, 2, 6, Pfizer, 6, Roche-Chugaï, 6, UCB, 6; B. Desta: AstraZeneca, 3; T. Grünfeld Eén: AstraZeneca, 3, 11; A. Sorrentino: AbbVie/Abbott, 12, Own stocks, AstraZeneca, 3, Galapagos, 12, Own stocks, Gilead, 12, Own stocks, Moderna, 12, Own stocks; S. Chen: AstraZeneca, 3, 11; B. Ding: AstraZeneca, 3.
Background/Purpose: The SLE Prospective Observational Cohort Study (SPOCS) is an international, multicenter study collecting data on patients with SLE, including in relation to oral corticosteroid (OCS) use and type 1 interferon gene signature (IFNGS) status. Here, we report SLICC/ACR Damage Index (DI) progression over 3 years in the overall population, as well as by baseline IFNGS category and average daily OCS use.
Methods: Eligible patients (aged ≥18 years) had physician-confirmed moderate-to-severe SLE (ACR or SLICC classification), a ≥6-month treatment duration with systemic SLE treatment beyond non-steroidal anti-inflammatory drugs and analgesics, and ≥1 lifetime positive serology of ANA or dsDNA. Disease organ damage burden was assessed using the SLICC/ACR DI score. IFNGS tests were performed in a central laboratory. The chi-square test was used to compare score categories and the proportions of patients with ≥1 new damage observation since the prior visit; the Kruskal–Wallis test was used for comparisons of the mean (standard deviation [SD]).
Results: Among 826 patients overall, 70.8% (n = 531) and 29.2% (n = 219) of patients had high and low IFNGS statuses, respectively; IFNGS status was missing for 76 patients. At baseline, 52.0% (n = 426) of patients grouped by high or low IFNGS status and 52.1% (n = 400) of patients grouped by OCS use had organ damage (DI score ≥1). The mean (SD) SLICC/ACR DI score was higher in patients with a low IFNGS than those with a high IFNGS; this trend continued throughout follow-up (Table 1). Average DI scores and percentages of patients with ≥1 new organ damage increased from baseline to 36 months in each category. Among patients stratified by daily OCS use (n = 768), 40.5% (n = 311), 44.0% (n = 338), and 15.5% (n = 119) of patients had no OCS use, low OCS use (0 – ≤7.5 mg/day), and high OCS use ( >7.5 mg/day) at baseline, respectively. Baseline mean (SD) DI scores were also similar in the overall, no OCS use, and low OCS use categories and higher in the high OCS use category (P = 0.209 for the no OCS use, low OCS use, and high OCS use categories; Table 2). The proportion of patients with ≥1 new organ damage increased from baseline to 12 months in each category (P = 0.611 for no OCS use, low OCS use, and high OCS use); a greater percentage of patients had ≥1 new organ damage in the high OCS category compared to the no or low OCS categories. Moreover, the mean (SD) DI score was higher in the high OCS category compared to the no OCS and low OCS categories after 12 months.
Conclusion: Results from the SPOCS study will enhance our understanding of the progression of SLE organ damage and will help improve clinical outcomes for patients with moderate-to-severe SLE. Nearly 20% of patients had new organ damage after 12 months. Patients with low IFNGS had greater organ damage than those with high IFNGS, and compared to no OCS or low daily average OCS use, organ damage is higher in patients with high average daily OCS use. Together, these indicate an unmet need for new treatment options for all patients with SLE, especially steroid-sparing treatments.

Table 1

Table 2
R. Furie: Biogen, 2, 5; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; C. Peschken: AstraZeneca, 2, 5, GSK, 2, 5, Roche, 1, 2; M. Aringer: AbbVie/Abbott, 1, 6, Boehringer-Ingelheim, 1, 6, Bristol-Myers Squibb(BMS), 1, 6, Chugai Pharma, 6, Eli Lilly, 1, 6, GlaxoSmithKlein(GSK), 1, 6, Merck/MSD, 6, Novartis, 1, Otsuka Pharma, 1, 6, Pfizer, 1, 6, Roche, 1, 6, Sanofi Aventis, 1, 6, UCB, 1, 6; L. Arnaud: AbbVie, 6, Alexion, 6, Alpine, 2, 6, Amgen, 6, AstraZeneca, 1, 2, 6, Biogen, 6, Boehringer Ingelheim, 6, Bristol-Myers Squibb(BMS), 2, 6, Eli Lilly, 6, GlaxoSmithKlein(GSK), 1, 2, 6, Grifols, 6, Janssen, 6, Kezar Life Sciences, 2, 6, LFB, 6, Medac, 6, Novartis, 2, 6, Pfizer, 6, Roche-Chugaï, 6, UCB, 6; B. Desta: AstraZeneca, 3; T. Grünfeld Eén: AstraZeneca, 3, 11; A. Sorrentino: AbbVie/Abbott, 12, Own stocks, AstraZeneca, 3, Galapagos, 12, Own stocks, Gilead, 12, Own stocks, Moderna, 12, Own stocks; S. Chen: AstraZeneca, 3, 11; B. Ding: AstraZeneca, 3.



