Poster Session C
Rheumatoid arthritis (RA)
Session: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
2126: Performance of the Rheumatoid Arthritis Impact of Disease (RAID) Score in Relation to Flares in Disease Activity
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Siri Lillegraven, MD, PhD, MPH
Diakonhjemmet Hospital
Oslo, NorwayDisclosure(s): Boehringer-Ingelheim: Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Karen Holten, Nina Paulshus Sundlisæter, Joseph Sexton, Lena Bugge Nordberg, Till Uhlig, Tore Kvien, Espen Haavardsholm, Siri Lillegraven and Anna-Birgitte Aga, Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway
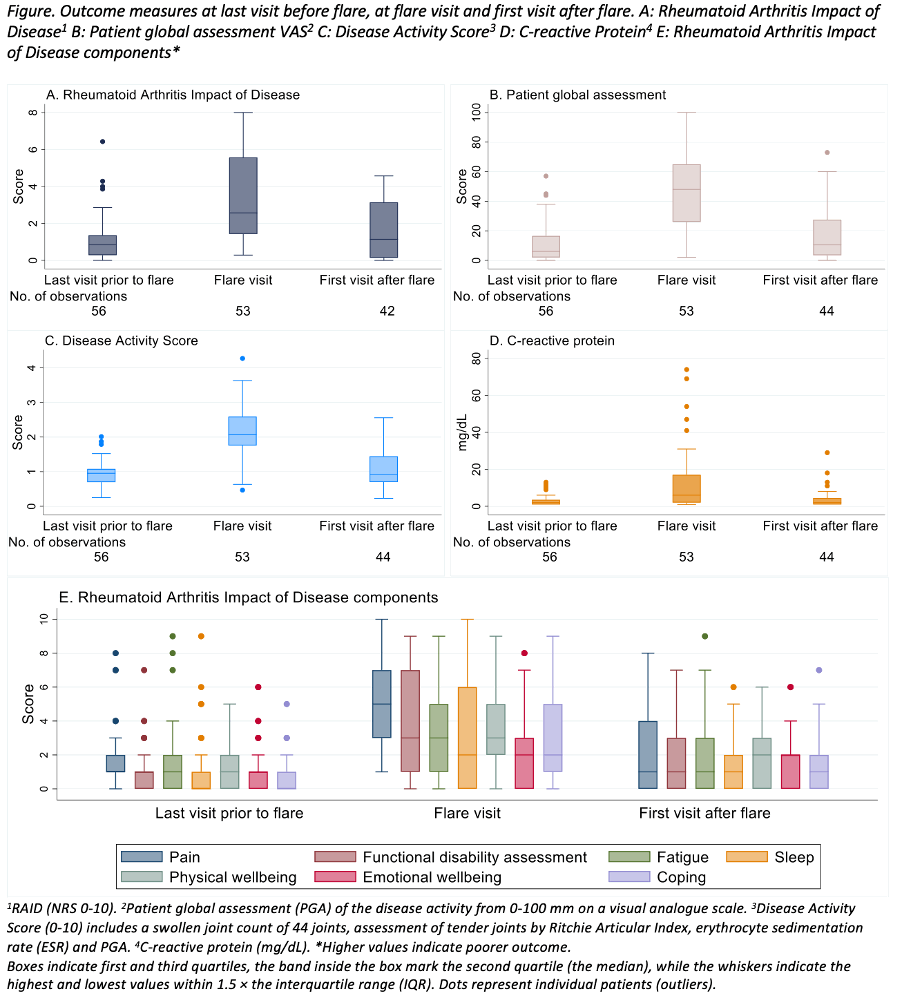
Background/Purpose: The Rheumatoid Arthritis Impact of Disease (RAID) is a patient-reported outcome measure (PROM) originating from a EULAR initiative. It might support patient-centred care and shared decision-making between patient and health care provider. RAID could be a useful tool in regular clinical settings and remote monitoring, and its association with disease activity is thus of interest. RAIDs performance with regard to flare is not known, and our aim was to explore its responsiveness and discriminative abilities with regard to flares in disease activity.
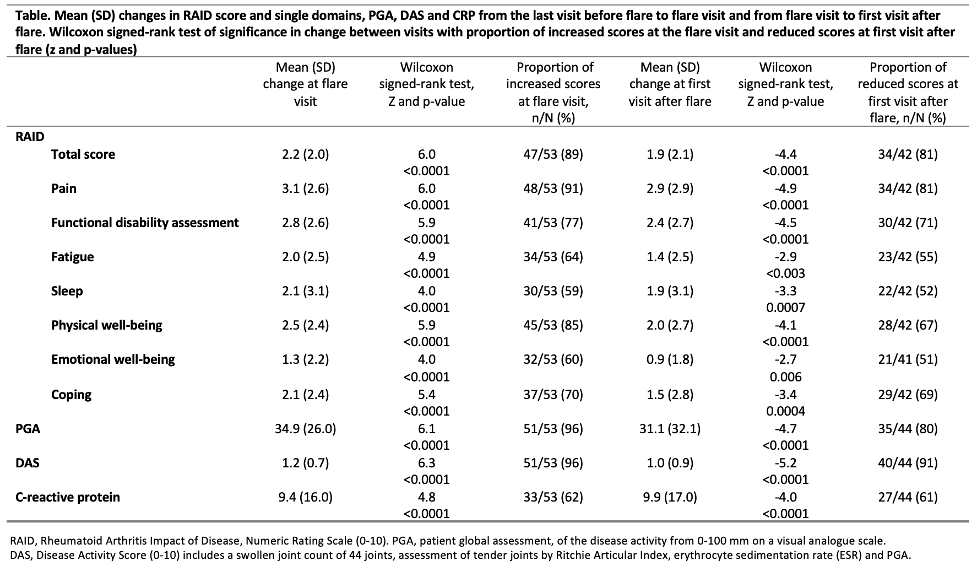
Methods: We used pooled data from the first 12 months of the two ARCTIC REWIND trials assessing tapering of TNFi and csDMARDs (ClinicalTrials.gov: NCT01881308) (Lillegraven et al, JAMA, 2021). Eligible participants had RA according to the ACR/EULAR 2010 criteria, were in sustained remission for ≥12 months on stable DMARDs with Disease Activity Score (DAS) remission combined with no swollen joints at inclusion. Patients were randomized to continued stable treatment or tapered treatment. Study visits were conducted every four months, if a flare was suspected between visits the patient was seen within a week. Flare was defined as a combination of DAS >1.6, a ≥0.6 units increase and minimum 2 swollen joints, or a flare could be recorded in consensus between patient and rheumatologist. We evaluated the median RAID (total score and components) at the last visit before flare, at the flare visit and first visit after flare in relation to the suggested ≤2 RAID threshold for patient acceptable symptom state (PASS), and assessed the changes between those visits using Wilcoxon signed-rank test. The discriminative accuracies of RAID (total score and components) with respect to flares were assessed using area under the ROC curve (AUC) analyses based on logistic regression models. For comparison, similar analyses were performed for patient global assessment (PGA), DAS and C-reactive protein (CRP).
Results: Of the 248 patients included, 159 (64%) were female. Mean (SD) age and disease duration were 56.1 (11.8) and 6.3 (5.7) years. For patients who experienced a flare the median (IQR) RAID score was 0.9 (0.3, 1.4) at last visit prior to flare with 86% of scores below the PASS threshold (Figure). At the flare visit median RAID was 2.6 (1.4, 5.6) with 70% of scores above the PASS. Similar changes were observed in DAS, PGA and CRP (Figure, Table). All seven RAID domains increased significantly at the flare visit (p-values < 0.001), with the largest increase in pain. AUC (95% CI) values were0.88 (0.83, 0.93) for RAID, 0.92 (0.87, 0.97) for PGA, 0.94 (0.90, 0.98) for DAS and 0.76 (0.69, 0.84) for CRP. The RAID components with highest and lowest discriminative accuracies were pain (0.91 (0.86 to 0.95)) and sleep (0.69 (0.59 to 0.79)).
Conclusion: Disease activity flares were associated with a significant increase in median RAID, supporting RAIDs ability to respond to flare. RAID and PGA discriminated well between flare and non-flare, hence both PROMs might contribute to identification of flare in a regular clinical setting as well as in remote monitoring of disease activity.
K. Holten: None; N. Sundlisæter: None; J. Sexton: None; L. Nordberg: None; T. Uhlig: Galapagos, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB, 2, 6; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; E. Haavardsholm: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Eli Lilly, 2, Gilead, 2, Pfizer, 6, UCB, 6; S. Lillegraven: Boehringer-Ingelheim, 5; A. Aga: AbbVie, 2, 6, Eli Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6.
Background/Purpose: The Rheumatoid Arthritis Impact of Disease (RAID) is a patient-reported outcome measure (PROM) originating from a EULAR initiative. It might support patient-centred care and shared decision-making between patient and health care provider. RAID could be a useful tool in regular clinical settings and remote monitoring, and its association with disease activity is thus of interest. RAIDs performance with regard to flare is not known, and our aim was to explore its responsiveness and discriminative abilities with regard to flares in disease activity.
Methods: We used pooled data from the first 12 months of the two ARCTIC REWIND trials assessing tapering of TNFi and csDMARDs (ClinicalTrials.gov: NCT01881308) (Lillegraven et al, JAMA, 2021). Eligible participants had RA according to the ACR/EULAR 2010 criteria, were in sustained remission for ≥12 months on stable DMARDs with Disease Activity Score (DAS) remission combined with no swollen joints at inclusion. Patients were randomized to continued stable treatment or tapered treatment. Study visits were conducted every four months, if a flare was suspected between visits the patient was seen within a week. Flare was defined as a combination of DAS >1.6, a ≥0.6 units increase and minimum 2 swollen joints, or a flare could be recorded in consensus between patient and rheumatologist. We evaluated the median RAID (total score and components) at the last visit before flare, at the flare visit and first visit after flare in relation to the suggested ≤2 RAID threshold for patient acceptable symptom state (PASS), and assessed the changes between those visits using Wilcoxon signed-rank test. The discriminative accuracies of RAID (total score and components) with respect to flares were assessed using area under the ROC curve (AUC) analyses based on logistic regression models. For comparison, similar analyses were performed for patient global assessment (PGA), DAS and C-reactive protein (CRP).
Results: Of the 248 patients included, 159 (64%) were female. Mean (SD) age and disease duration were 56.1 (11.8) and 6.3 (5.7) years. For patients who experienced a flare the median (IQR) RAID score was 0.9 (0.3, 1.4) at last visit prior to flare with 86% of scores below the PASS threshold (Figure). At the flare visit median RAID was 2.6 (1.4, 5.6) with 70% of scores above the PASS. Similar changes were observed in DAS, PGA and CRP (Figure, Table). All seven RAID domains increased significantly at the flare visit (p-values < 0.001), with the largest increase in pain. AUC (95% CI) values were0.88 (0.83, 0.93) for RAID, 0.92 (0.87, 0.97) for PGA, 0.94 (0.90, 0.98) for DAS and 0.76 (0.69, 0.84) for CRP. The RAID components with highest and lowest discriminative accuracies were pain (0.91 (0.86 to 0.95)) and sleep (0.69 (0.59 to 0.79)).
Conclusion: Disease activity flares were associated with a significant increase in median RAID, supporting RAIDs ability to respond to flare. RAID and PGA discriminated well between flare and non-flare, hence both PROMs might contribute to identification of flare in a regular clinical setting as well as in remote monitoring of disease activity.


K. Holten: None; N. Sundlisæter: None; J. Sexton: None; L. Nordberg: None; T. Uhlig: Galapagos, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB, 2, 6; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; E. Haavardsholm: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Eli Lilly, 2, Gilead, 2, Pfizer, 6, UCB, 6; S. Lillegraven: Boehringer-Ingelheim, 5; A. Aga: AbbVie, 2, 6, Eli Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6.



