Poster Session C
Rheumatoid arthritis (RA)
Session: (2141–2176) RA – Treatment Poster III
2169: Cost-utility of a Progressive Spacing of Tocilizumab or Abatacept in Patients with Rheumatoid Arthritis in Sustained Remission: A Medico-economic Analysis of the Towards the Lowest Efficacious Dose Trial
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Bruno Fautrel, MD, PhD
Sorbonne University - APHP
Paris, FranceDisclosure information not submitted.
Abstract Poster Presenter(s)
Joanna Kedra1, Benjamin Granger2, Lina El Houari3, Florence Tubach4 and Bruno Fautrel5, 1Sorbonne Université, IPLESP, and Pitié-Salpêtrière Hospital, Paris, France, 2Sorbonne Université, INSERM, and Pitié Salpêtrière Hospital, Paris, France, 3Institut Pierre Louis d’Epidémiologie et de Santé Publique, Paris, France, 4Centre de pharmaco-épidémiologie de l'APHP, Paris, France, 5Sorbonne Université APHP, Paris, France
Background/Purpose: Biologic Disease Modifying Anti-Rheumatic Drugs (bDMARDs) progressive tapering is a real opportunity in people living with rheumatoid arthritis (RA) having achieved remission both from the patient (reduction in the disease and drug-related burden) and the Society (cost alleviation) perspectives. The ToLEDo (Towards the Lowest Efficacious Dose) trial aimed to assess a disease activity-driven progressive tapering strategy of tocilizumab (TCZ) or abatacept (ABA) compared to their maintenance at full dose in RA patients in sustained remission. Non-inferiority (NI) was not demonstrated in terms of disease activity nor relapses, major relapses, radiographic progression. The aim of this secondary analysis was to assess the cost-utility of the spacing strategy (S-arm) in the ToLEDo trial compared to full dose maintenance (M-arm).
Methods: ToLEDo is a multicenter 2-year NI randomized open-label controlled trial, which enrolled 228 patients (113 in the S-arm and 115 in the M-arm). A cost-utility analysis was conducted on the per protocol population. In each arm, health benefits were estimated every 6 months by Short Form Health Survey (SF-6D) and EuroQoL (EQ-5D)-derived utility measurements. Cost elicitation integrated health resource use including bDMARD costs (direct cost) as well as productivity loss (indirect cost).The incremental cost-utility ratios (ICUR) were calculated by dividing the difference of costs between S-arm and M-arm by the difference of utilities between the 2 arms. 95% confidence interval (95%CI) were calculated by bootstrap (20,000 iterations). The incremental net benefit (INB) was calculated for willingness to pay (WTP) values ranging from 0 to 150,000€. The analyses were replicated using SF-6D (primary analysis) or EQ-5D, and in ABA and TCZ subgroups. Acceptability analyses as well as stochastic sensitivity analyses (simulating costs and utilities using MCMC algorithms) were also performed.
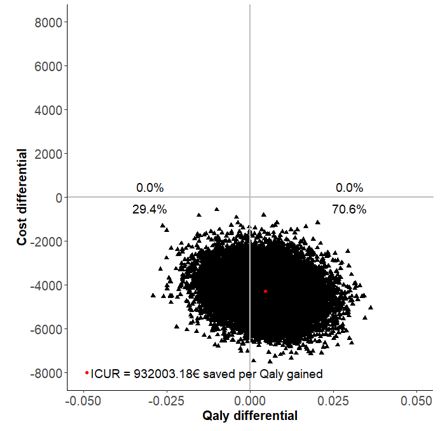
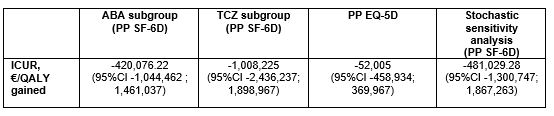
Results: Overall, 178 patients were included (82 in S-arm, 96 in M-arm) in the per protocol analysis. At the end of the follow-up in the S-arm, 15.0% of patients discontinued their biologic, 48.7% spaced the injections, and 36.3% remained at the standard dose. The difference in terms of two-years utility gains between S-arm and M-arm was 0.004 (95%CI -0.012, 0.021) with SF-6D. The difference of total costs between S-arm and M-arm was -4,275 € (95%CI -5,955 to -2,542). The estimated ICUR of the spacing strategy over the maintenance at full dose was €932,003 saved per QALY (95% CI -7,534,788 to 6,720,372) with SF-6D. The INB was 4,734.6€ for a WTP of 100,000€. With a willingness to accept of 0 €/QALY lost, the probability to be cost-effective for the spacing strategy was 70.6% (Figure 1). The results were consistent when using EQ-5D-derived utilities, in ABA and TCZ subgroups, as well as in the stochastic sensitivity analyses (Table 1).
Conclusion: Although the ToLEDo trial did not demonstrate non-inferiority, the tested disease activity-driven tapering strategy was not associated with health loss in terms of utilities and incurred for substantial cost savings, making this strategy potentially dominant.
J. Kedra: Amgen, 12, Hospitality, Bristol-Myers Squibb(BMS), 6, Galapagos, 2, Roche, 2; B. Granger: Bristol-Myers Squibb(BMS), 2; L. El Houari: None; F. Tubach: Lundbeck, 2, Merck/MSD, 2, UCB, 2; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2.
Background/Purpose: Biologic Disease Modifying Anti-Rheumatic Drugs (bDMARDs) progressive tapering is a real opportunity in people living with rheumatoid arthritis (RA) having achieved remission both from the patient (reduction in the disease and drug-related burden) and the Society (cost alleviation) perspectives. The ToLEDo (Towards the Lowest Efficacious Dose) trial aimed to assess a disease activity-driven progressive tapering strategy of tocilizumab (TCZ) or abatacept (ABA) compared to their maintenance at full dose in RA patients in sustained remission. Non-inferiority (NI) was not demonstrated in terms of disease activity nor relapses, major relapses, radiographic progression. The aim of this secondary analysis was to assess the cost-utility of the spacing strategy (S-arm) in the ToLEDo trial compared to full dose maintenance (M-arm).
Methods: ToLEDo is a multicenter 2-year NI randomized open-label controlled trial, which enrolled 228 patients (113 in the S-arm and 115 in the M-arm). A cost-utility analysis was conducted on the per protocol population. In each arm, health benefits were estimated every 6 months by Short Form Health Survey (SF-6D) and EuroQoL (EQ-5D)-derived utility measurements. Cost elicitation integrated health resource use including bDMARD costs (direct cost) as well as productivity loss (indirect cost).The incremental cost-utility ratios (ICUR) were calculated by dividing the difference of costs between S-arm and M-arm by the difference of utilities between the 2 arms. 95% confidence interval (95%CI) were calculated by bootstrap (20,000 iterations). The incremental net benefit (INB) was calculated for willingness to pay (WTP) values ranging from 0 to 150,000€. The analyses were replicated using SF-6D (primary analysis) or EQ-5D, and in ABA and TCZ subgroups. Acceptability analyses as well as stochastic sensitivity analyses (simulating costs and utilities using MCMC algorithms) were also performed.
Results: Overall, 178 patients were included (82 in S-arm, 96 in M-arm) in the per protocol analysis. At the end of the follow-up in the S-arm, 15.0% of patients discontinued their biologic, 48.7% spaced the injections, and 36.3% remained at the standard dose. The difference in terms of two-years utility gains between S-arm and M-arm was 0.004 (95%CI -0.012, 0.021) with SF-6D. The difference of total costs between S-arm and M-arm was -4,275 € (95%CI -5,955 to -2,542). The estimated ICUR of the spacing strategy over the maintenance at full dose was €932,003 saved per QALY (95% CI -7,534,788 to 6,720,372) with SF-6D. The INB was 4,734.6€ for a WTP of 100,000€. With a willingness to accept of 0 €/QALY lost, the probability to be cost-effective for the spacing strategy was 70.6% (Figure 1). The results were consistent when using EQ-5D-derived utilities, in ABA and TCZ subgroups, as well as in the stochastic sensitivity analyses (Table 1).
Conclusion: Although the ToLEDo trial did not demonstrate non-inferiority, the tested disease activity-driven tapering strategy was not associated with health loss in terms of utilities and incurred for substantial cost savings, making this strategy potentially dominant.

Figure 1: cost-utility plane (spacing versus maintenance), with utilities derived from PP SF-6D

Table 1: ICUR in ABA subgroup, TCZ subgroup, using EQ-5D-derived utilities, and stochastic sensitivity analysis
J. Kedra: Amgen, 12, Hospitality, Bristol-Myers Squibb(BMS), 6, Galapagos, 2, Roche, 2; B. Granger: Bristol-Myers Squibb(BMS), 2; L. El Houari: None; F. Tubach: Lundbeck, 2, Merck/MSD, 2, UCB, 2; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2.



