Poster Session C
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (1945–1972) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
1965: Discordance Between Patient and Physician Perception of Disease Activity Among Patients with Idiopathic Inflammatory Myopathies: Results from the COVAD Study
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MD
Mrinalini Dey, MRCP, MA, MBBCH
Centre for Rheumatic Diseases, King's College London
London, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Mrinalini Dey1, Rudra Prosad Goswami2, Parikshit Sen3, Samuel Shinjo4, Nelly Ziade5, Ioannis Parodis6, Mrudula Joshi7, Marcin Milchert8, Abraham Edgar Gracia-Ramos9, Lorenzo Cavagna10, Vishwesh Agarwal11, Johannes Knitza12, Jessica Day13, Hector Chinoy14, COVAD Study Group15, Vikas Agarwal16, Rohit Aggarwal17 and Latika Gupta18, 1Queen Elizabeth Hospital, London, United Kingdom; University of Liverpool, Liverpool, United Kingdom, 2Department of Rheumatology, All India Institute of Medical Sciences, New Delhi, India, 3Maulana Azad Medical College, 2-Bahadurshah Zafar Marg, New Delhi, Delhi-110002, India., Dalhi, India, 4Faculdade de Medicina FMUSP, Universidade de Sao Paulo, São Paulo, Brazil, 5Saint-Joseph University, Beirut, Lebanon, 6Karolinska Institutet, Stockholm, Sweden, 7Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, India, 8Department of Internal Medicine, Rheumatology, Diabetology, Geriatrics and Clinical Immunology, Pomeranian Medical University in Szczecin, Szczecin, Poland, 9Department of Internal Medicine, General Hospital, National Medical Center "La Raza", Instituto Mexicano del Seguro Social, Av. Jacaranda S/N, Col. La Raza, Del. Azcapotzalco, C.P. 02990, Mexico City, Mexico, 10Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, 11Mahatma Gandhi Missions Medical College, Lucknow, India, 12Department of Internal Medicine 3 Rheumatology and Immunology, Friedrich-Alexander-University Erlangen-Nürnberg, University Hospital Erlangen, Erlangen, Germany, 13Walter and Eliza Hall Institute, Melbourne, Australia, 14The University of Manchester, Sale, United Kingdom, 15-, -, 16Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, India, 17University of Pittsburgh, Pittsburgh, PA, 18Royal Wolverhampton Trust, Wolverhampton/University of Manchester, United Kingdom
Background/Purpose: Disease activity assessment is key in the management of patients with idiopathic inflammatory myopathies (IIM). However, patients' perception of disease may differ from clinicians. We tried to understand what factors lead to discrepancy between patients' and physicians' perception of disease activity in IIMs from the COVID-19 Vaccination in Autoimmune Diseases (COVAD) study.
Methods: The COVAD-1 study collected global cross-sectional data from adults with autoimmune diseases and healthy controls by a team of 106 experts from 94 countries from April-December 2021, on demographics; disease characteristics, treatment and glucocorticoid (GC) dose; and validated patient reported outcomes (PROs), including PROMIS SF10 physical function, VAS-pain and VAS-fatigue. Patient-perceived active disease was self-reported. Physician-reported active disease was defined as presence of joint swelling, active rash or worsened muscle weakness and/or GC dose ≥10mg prednisolone equivalents. Discordant and concordant pairs of patient-physician reported disease activity were compared, and predictors of discordance were analysed using multivariate regression models.
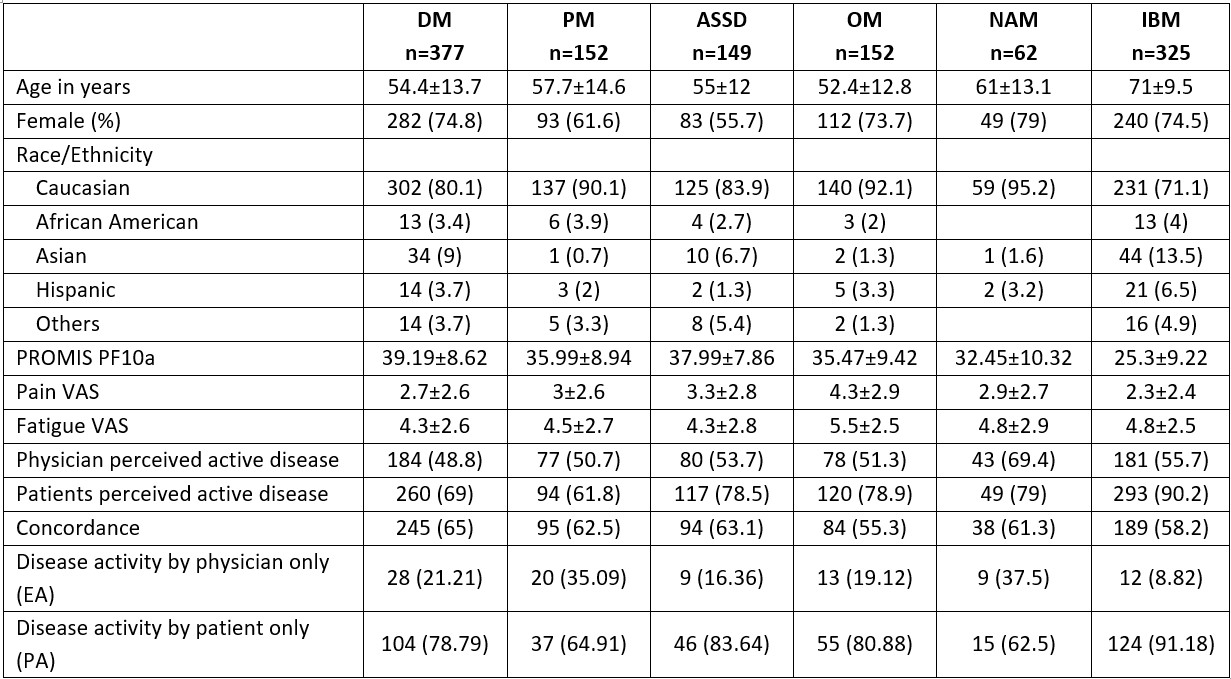
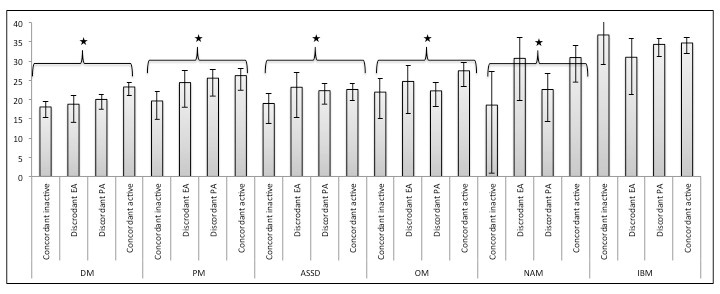
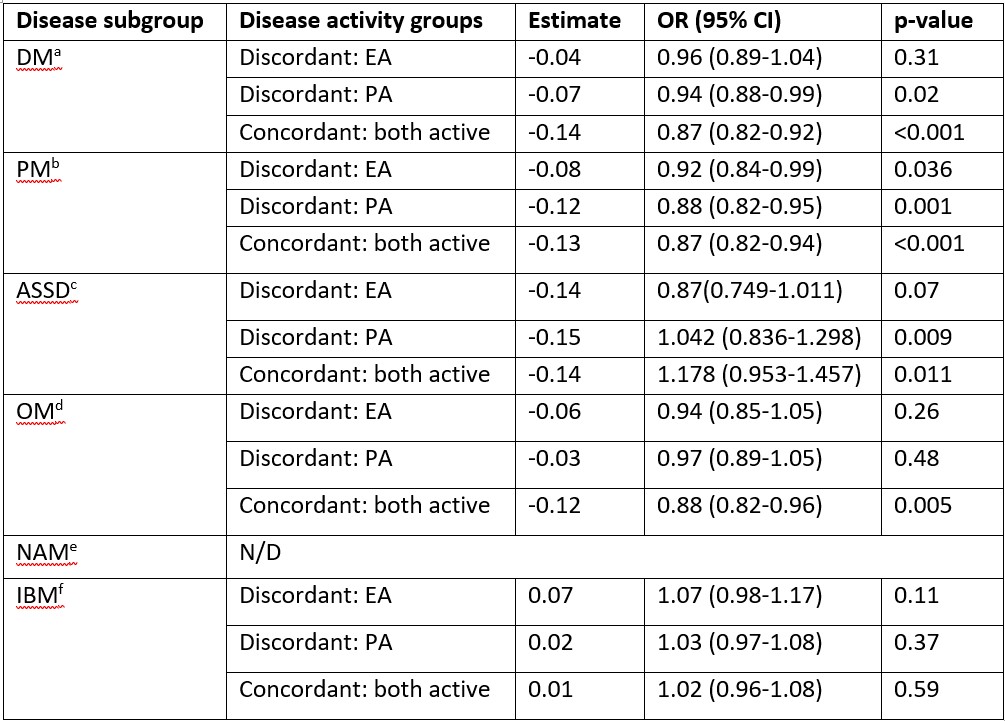
Results: Among 16328 respondents, 1217 patients with IIM were included (median age 59 years (IQR 45-73), 70% females and 81.6% Caucasians, details in Table 1). Dermatomyositis (DM; 30.9%) was the most common subtype. Moderate concordance was seen between patient and physician disease activity. Discordance was identified in 38.8% (n=472) responses. Poorest concordance was observed in inclusion body myositis (IBM), overlap myositis (OM) and necrotising autoimmune myopathy (NAM) subgroups. Among patients with DM and OM, those with higher PROMIS physical function scores (i.e. better physical function) were more likely to be concordant (DM: OR=1.03, 95% CI: 1.01-1.05, p=0.012; OM: OR=1.04, 95% CI: 1.01-1.06, p=0.012) while older patients with DM were prone to discordance (OR =0.98, 95% CI: 0.97-0.99, p=0.03) PROMIS scores varied significantly among the four groups of perceived disease activity (active or inactive by patient and/or physician) and disease subgroups except IBM (Figure 1). Pain and Fatigue VAS scores also varied significantly among the four groups. Multivariable analysis, controlling for age, gender, fatigue and pain, showed respondents with higher PROMIS physical function scores were more likely to belong to one of the active disease perception groups, compared to concordant inactive groups, for all disease subgroups except IBM (Table 2). Addition of PROMIS increased area under the curve by 8-10% in model fitting. Simple cut-off scores based on PROMIS scores could be developed to distinguish active from inactive disease.
Conclusion: Our study provides invaluable insights into the factors determining patents' perception of self-reported disease activity, and the disease groups where these measures may be less reliable for remote monitoring and virtual trials. Patient-reported assessment might be discordant in IBM while likely to be fairly concordant in DM and younger individuals irrespective of pain and fatigue.
M. Dey: None; R. Goswami: None; P. Sen: None; S. Shinjo: None; N. Ziade: Abbvie, 6, Boehringer-Ingelheim, 6, Eli Lilly, 6, Janssen, 6, Newbridge, 6, Novartis, 6, Pfizer, 6, Pierre Fabre, 6, Roche, 6, sanofi, 6; I. Parodis: Amgen, 5, 6, AstraZeneca, 5, 6, Aurinia Pharmaceuticals, 5, 6, Bristol-Myers Squibb(BMS), 5, 6, Elli Lilly and Company, 5, 6, F. Hoffmann-La Roche AG, 5, 6, Gilead Sciences, 5, 6, GSK, 5, 6, Janssen Pharmaceuticals, 5, 6, Novartis, 5, 6, Otsuka Pharmaceutical, 5, 6; M. Joshi: None; M. Milchert: None; A. Gracia-Ramos: None; L. Cavagna: None; V. Agarwal: None; J. Knitza: None; J. Day: CSL limited, 5; H. Chinoy: AstraZeneca, 1, Biogen, 2, Eli Lilly, 5, GlaxoSmithKlein(GSK), 6, Novartis, 2, Orphazyme, 2, Pfizer, 1, UCB, 6; C. Study Group: None; V. Agarwal: None; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2; L. Gupta: None.
Background/Purpose: Disease activity assessment is key in the management of patients with idiopathic inflammatory myopathies (IIM). However, patients' perception of disease may differ from clinicians. We tried to understand what factors lead to discrepancy between patients' and physicians' perception of disease activity in IIMs from the COVID-19 Vaccination in Autoimmune Diseases (COVAD) study.
Methods: The COVAD-1 study collected global cross-sectional data from adults with autoimmune diseases and healthy controls by a team of 106 experts from 94 countries from April-December 2021, on demographics; disease characteristics, treatment and glucocorticoid (GC) dose; and validated patient reported outcomes (PROs), including PROMIS SF10 physical function, VAS-pain and VAS-fatigue. Patient-perceived active disease was self-reported. Physician-reported active disease was defined as presence of joint swelling, active rash or worsened muscle weakness and/or GC dose ≥10mg prednisolone equivalents. Discordant and concordant pairs of patient-physician reported disease activity were compared, and predictors of discordance were analysed using multivariate regression models.
Results: Among 16328 respondents, 1217 patients with IIM were included (median age 59 years (IQR 45-73), 70% females and 81.6% Caucasians, details in Table 1). Dermatomyositis (DM; 30.9%) was the most common subtype. Moderate concordance was seen between patient and physician disease activity. Discordance was identified in 38.8% (n=472) responses. Poorest concordance was observed in inclusion body myositis (IBM), overlap myositis (OM) and necrotising autoimmune myopathy (NAM) subgroups. Among patients with DM and OM, those with higher PROMIS physical function scores (i.e. better physical function) were more likely to be concordant (DM: OR=1.03, 95% CI: 1.01-1.05, p=0.012; OM: OR=1.04, 95% CI: 1.01-1.06, p=0.012) while older patients with DM were prone to discordance (OR =0.98, 95% CI: 0.97-0.99, p=0.03) PROMIS scores varied significantly among the four groups of perceived disease activity (active or inactive by patient and/or physician) and disease subgroups except IBM (Figure 1). Pain and Fatigue VAS scores also varied significantly among the four groups. Multivariable analysis, controlling for age, gender, fatigue and pain, showed respondents with higher PROMIS physical function scores were more likely to belong to one of the active disease perception groups, compared to concordant inactive groups, for all disease subgroups except IBM (Table 2). Addition of PROMIS increased area under the curve by 8-10% in model fitting. Simple cut-off scores based on PROMIS scores could be developed to distinguish active from inactive disease.
Conclusion: Our study provides invaluable insights into the factors determining patents' perception of self-reported disease activity, and the disease groups where these measures may be less reliable for remote monitoring and virtual trials. Patient-reported assessment might be discordant in IBM while likely to be fairly concordant in DM and younger individuals irrespective of pain and fatigue.

Table 1. Demographics and patient reported outcome measures among the disease subgroups. ASSD: anti-synthetase syndrome; DM: dermatomyositis; IBM: inclusion body myositis; NAM: Immune mediated necrotising myopathy; OM: overlap myositis; PF: physical function; PM: polymyositis; PROMIS, Patient-Reported Outcome Information System; VAS: visual analogue scale (0-10).

Figure 1. Distribution of the mean PROMIS PF10a scores according to the four perceived disease activity classes among the different disease subgroups. *= p value<0.001 by the Kruskal Wallis test.
Abbreviations: ASSD: anti-synthetase syndrome; DM: dermatomyositis; EA: evaluator assessment as active disease; IBM: inclusion body myositis; NAM: Immune mediated necrotising myopathy; OM: overlap myositis; PA: patient assessment as active disease; PF: physical function; PM: polymyositis; PROMIS, Patient-Reported Outcome Information System.
Abbreviations: ASSD: anti-synthetase syndrome; DM: dermatomyositis; EA: evaluator assessment as active disease; IBM: inclusion body myositis; NAM: Immune mediated necrotising myopathy; OM: overlap myositis; PA: patient assessment as active disease; PF: physical function; PM: polymyositis; PROMIS, Patient-Reported Outcome Information System.

Table 2. Multivariable regression analysis with the four groups of perceived disease activity as dependent variable.
Only the parameter estimates of PROMISPF10a scores as the independent variables is shown in the table. The model was adjusted with the following covariates: age, gender, Fatigue VAS and pain VAS. a Other significant predictors: Pain for “Discordant PA”: 1.27 (1.01-1.16), p=0.04; Fatigue for “Both active”: 1.16 (1.002-1.33), p=0.048; b Other significant predictors: None; c Other significant predictors: None; d Other significant predictors: Age for “Discordant: PA”: 0.94 (0.90-0.99), p=0.023 and for “Concordant: Both active”: 0.94 (0.90-0.99), p=0.02; e Not fitted due to very low sample size in reference category; f Other significant predictors: None.
Abbreviations: ASSD: anti-synthetase syndrome; CI: confidence interval; DM: dermatomyositis; EA: only physician’s perceives disease as active and patient does not; IBM: inclusion body myositis; NAM: Immune mediated necrotising myopathy; N/D: Not done; OM: overlap myositis; OR: Odd’s ratio; PA: only patient perceives disease as active and physician doses not; PF: physical function; PM: polymyositis; PROMIS, Patient-Reported Outcome Information System; VAS: visual analogue scale (0-10).
Only the parameter estimates of PROMISPF10a scores as the independent variables is shown in the table. The model was adjusted with the following covariates: age, gender, Fatigue VAS and pain VAS. a Other significant predictors: Pain for “Discordant PA”: 1.27 (1.01-1.16), p=0.04; Fatigue for “Both active”: 1.16 (1.002-1.33), p=0.048; b Other significant predictors: None; c Other significant predictors: None; d Other significant predictors: Age for “Discordant: PA”: 0.94 (0.90-0.99), p=0.023 and for “Concordant: Both active”: 0.94 (0.90-0.99), p=0.02; e Not fitted due to very low sample size in reference category; f Other significant predictors: None.
Abbreviations: ASSD: anti-synthetase syndrome; CI: confidence interval; DM: dermatomyositis; EA: only physician’s perceives disease as active and patient does not; IBM: inclusion body myositis; NAM: Immune mediated necrotising myopathy; N/D: Not done; OM: overlap myositis; OR: Odd’s ratio; PA: only patient perceives disease as active and physician doses not; PF: physical function; PM: polymyositis; PROMIS, Patient-Reported Outcome Information System; VAS: visual analogue scale (0-10).
M. Dey: None; R. Goswami: None; P. Sen: None; S. Shinjo: None; N. Ziade: Abbvie, 6, Boehringer-Ingelheim, 6, Eli Lilly, 6, Janssen, 6, Newbridge, 6, Novartis, 6, Pfizer, 6, Pierre Fabre, 6, Roche, 6, sanofi, 6; I. Parodis: Amgen, 5, 6, AstraZeneca, 5, 6, Aurinia Pharmaceuticals, 5, 6, Bristol-Myers Squibb(BMS), 5, 6, Elli Lilly and Company, 5, 6, F. Hoffmann-La Roche AG, 5, 6, Gilead Sciences, 5, 6, GSK, 5, 6, Janssen Pharmaceuticals, 5, 6, Novartis, 5, 6, Otsuka Pharmaceutical, 5, 6; M. Joshi: None; M. Milchert: None; A. Gracia-Ramos: None; L. Cavagna: None; V. Agarwal: None; J. Knitza: None; J. Day: CSL limited, 5; H. Chinoy: AstraZeneca, 1, Biogen, 2, Eli Lilly, 5, GlaxoSmithKlein(GSK), 6, Novartis, 2, Orphazyme, 2, Pfizer, 1, UCB, 6; C. Study Group: None; V. Agarwal: None; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2; L. Gupta: None.



