Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III: SpA
2245: Drug Survival of Risankizumab vs Other Biologics After 13 Months of Treatment Among Patients with PsA in the Multicountry Postmarketing Observational VALUE Study
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- RL
Abstract Poster Presenter(s)
Lars Erik1, Kim A Papp2, Andrew Östör3, Vassilis Stakias4, Tshepiso Madihlaba4, Ralph Lippe5, Ran Liu4 and Diamant Thaçi6, 1Bispebjerg-Frederiksberg Hospital, Vedbæk, Denmark, 2Alliance Clinical Research and Probity Medical Research, Waterloo, and University of Toronto, Toronto, ON, Canada, 3Monash University & Emeritus Research; Australian National University, Melbourne, Australia, 4AbbVie, Inc., North Chicago, IL, 5AbbVie Deutschland GmbH & Co. KG, Wiesbaden, Germany, 6Institute and Comprehensive Center for Inflammation Medicine, University of Lübeck, Lübeck, Germany
Background/Purpose: Risankizumab (RZB) is an optimized IL-23 inhibitor (IL-23i) currently approved for the treatment of plaque psoriasis (PsO), PsA, and Crohn's disease. In a post hoc analysis of data from the postmarketing VALUE study in patients with PsO who were treated with RZB or other biologic therapies, we assessed real‑world drug survival for a subgroup of patients also diagnosed with PsA.
Methods: The ongoing, multicountry, prospective observational cohort VALUE study (NCT03982394) enrolled adults (aged ≥ 18 years) who were diagnosed with moderate-to-severe PsO and prescribed RZB or another biologic therapy in accordance with local labeling in a 2:1 ratio. All treatment decisions were made solely by the treating physician. This post hoc analysis included data from the subset of patients with PsO who were also diagnosed with PsA by a rheumatologist and was based on an interim database lock (data cutoff, September 26, 2022). Drug survival was compared for patients receiving RZB vs all other biologics, vs biologics excluding IL-23i, vs TNF-α inhibitors (TNFi), and vs IL-17 inhibitors (IL‑17i). The probability of drug survival was estimated from Kaplan‑Meier curves of time to first drug change (defined as discontinuation of the prescribed biologic treatment or changing to a different biologic treatment). Chi-squared tests were used to evaluate the proportion of patients who had drug changes by month 13. Analyses were repeated after propensity score matching (PSM) with a 1:1 ratio using greedy algorithm and exact match for biologic-naive/biologic-experienced status to account for baseline imbalances between comparison groups.
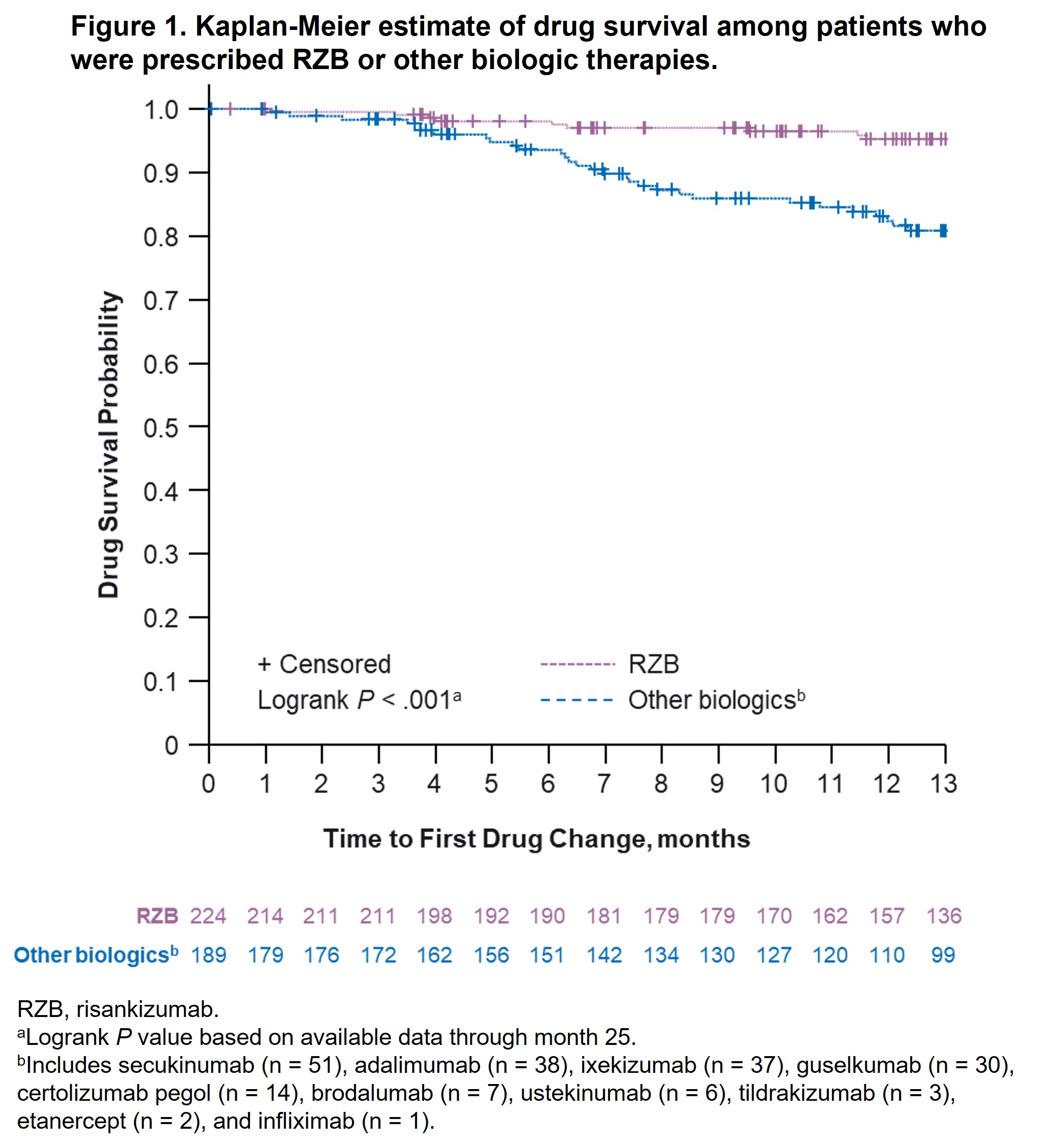
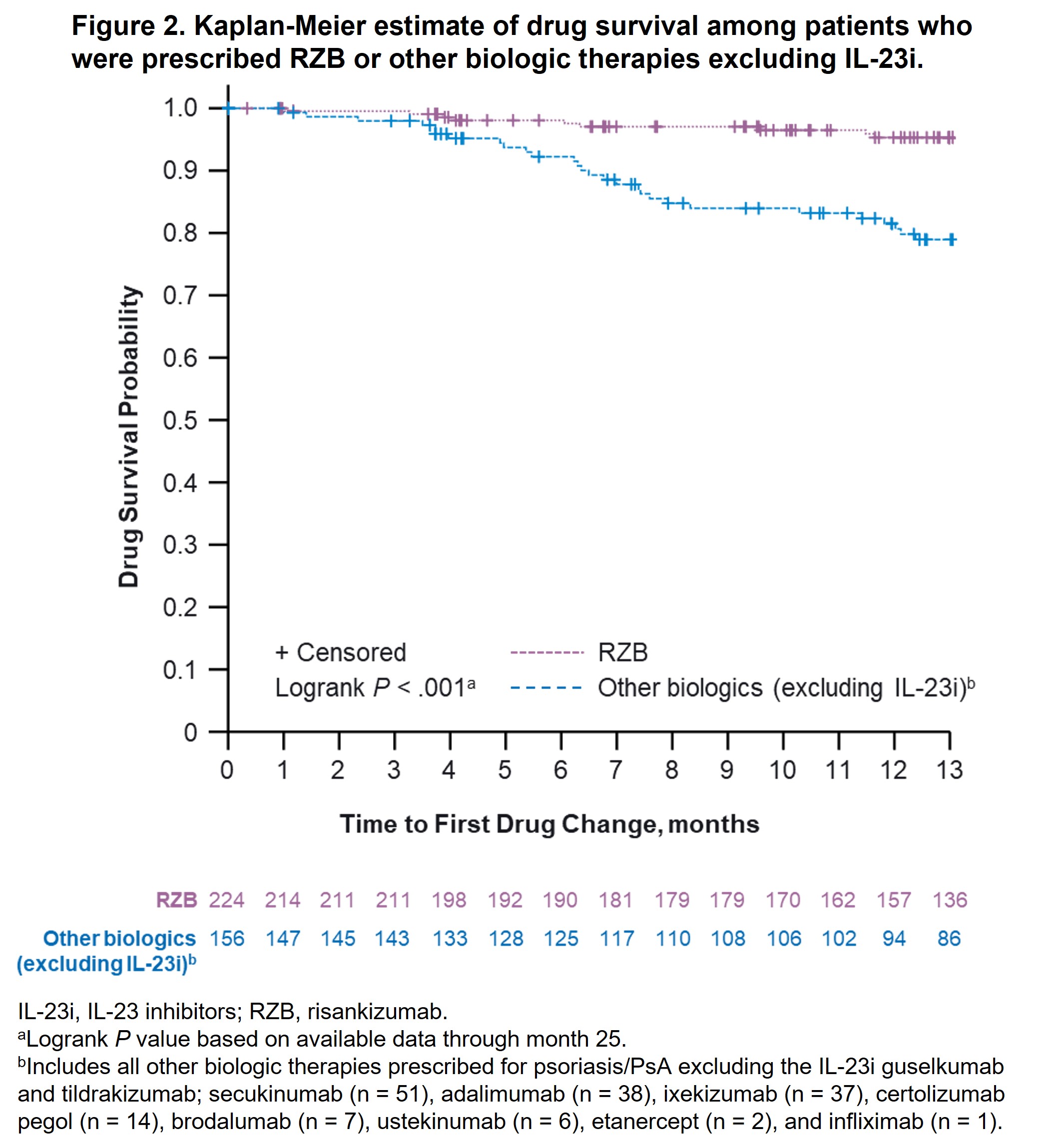
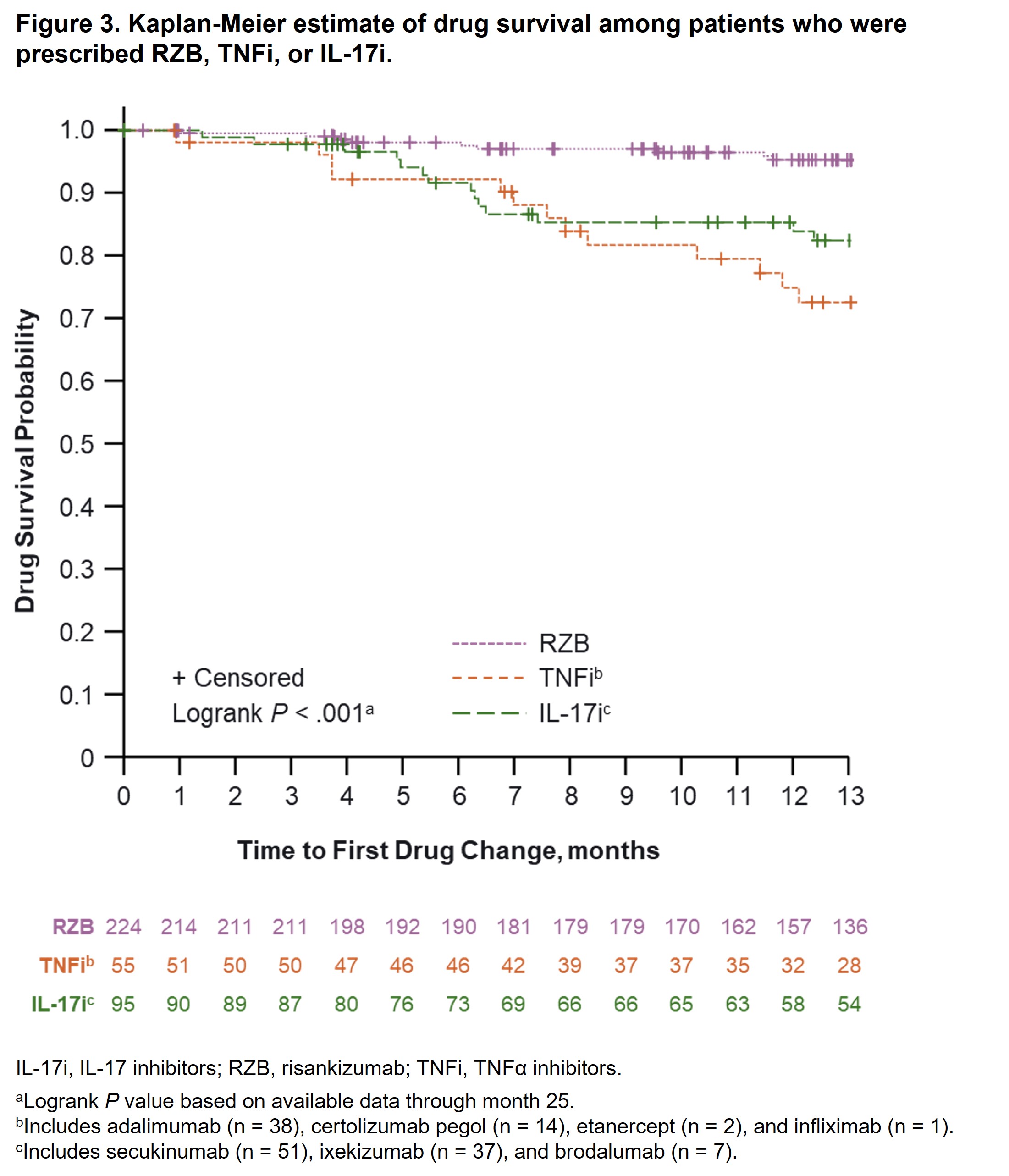
Results: A total of 244 (RZB) and 189 (other biologics) patients diagnosed with PsO and PsA were included in this analysis. Baseline demographics and characteristics were generally comparable between groups, except patients prescribed RZB vs other biologics were older (median age, 55 vs 51 years, P = .02) and more likely to have prior biologic therapy experience (67.4% vs 43.4%, P < .001). At month 13, fewer patients prescribed RZB had drug changes (5.5%) vs patients prescribed other biologics (19.0%), other biologics excluding IL-23i (20.5%), TNFi (26.7%), and IL‑17i (16.4%; P < .001 for all comparisons). The estimated probability of drug survival [95% CI] at 13 months was higher among patients receiving RZB (95.3% [91.1%, 97.5%]) vs other biologics (80.9% [73.7%, 86.3%]; Figure 1); RZB vs biologics excluding IL-23i (78.9% [70.9%, 85.0%]; Figure 2), or RZB vs TNFi and IL-17i (72.5% [57.3%, 83.1%] and 82.4% [72.0%, 89.2%]; Figure 3). Similar results were observed after PSM (estimated probability of drug survival [95% CI] at 13 months for RZB, 94.8% [88.7%, 97.6%]; other biologics, 84.8% [76.3%, 90.4%]; biologics excluding IL-23i, 82.4% [72.4%, 89.0%]; TNFi, 82.7% [63.2%, 92.4%]; and IL‑17i, 82.8% [69.5%, 90.7%]).
Conclusion: Patients with PsO and PsA who were treated with RZB in the real-world setting experienced fewer drug changes and a higher probability of drug survival compared with those treated with other biologic therapies. These results can help inform clinician decision making when initiating biologic treatment in patients with PsA.
L. Erik: AbbVie, 2, 5, 6, Amgen, 2, 6, Biogen, 2, 6, Bristol-Myers Squibb, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 2, 6, Janssen, 2, 6, MSD, 2, 6, Novartis, 2, 5, 6, Pfizer, 2, 6, UCB, 2, 5, 6; K. Papp: AbbVie, 1, 2, 5, 6, Akros, 1, 2, 5, 6, Amgen, 1, 2, 5, 6, Anacor, 1, 2, 5, 6, Arcutis, 1, 2, 5, 6, Astellas, 1, 2, 5, 6, Bausch Health/Valeant, 1, 2, 5, 6, Baxalta, 1, 2, 5, 6, Boehringer-Ingelheim, 1, 2, 5, 6, Bristol-Myers Squibb, 1, 2, 5, 6, Can-Fite Biopharma, 1, 2, 5, 6, Celgene, 1, 2, 5, 6, Coherus, 1, 2, 5, 6, Dermira, 1, 2, 5, 6, Dow Pharma, 1, 2, 5, 6, Eli Lilly, 1, 2, 5, 6, Evelo, 1, 2, 5, 6, Forward Pharma, 5, Galapagos, 1, 2, 5, 6, Galderma, 1, 2, 5, 6, Genentech, 1, 2, 5, 6, Gilead, 1, 2, 5, 6, GlaxoSmithKlein, 1, 2, 5, 6, Janssen, 1, 2, 5, 6, Kyowa-Hakko Kirin, 1, 2, 5, 6, LEO Pharma, 1, 2, 5, 6, MedImmune, 1, 2, 5, 6, Meiji Seika Pharma, 1, 2, 5, 6, Merck-Serono, 1, 2, 5, 6, Mitsubishi Pharma, 1, 2, 5, 6, Moberg Pharma, 1, 2, 5, 6, MSD, 1, 2, 5, 6, Novartis, 1, 2, 5, 6, Pfizer, 1, 2, 5, 6, PRCL Research, 1, 2, 5, 6, Regeneron, 1, 2, 5, 6, Roche, 1, 2, 5, 6, Sanofi-Aventis/Genzyme, 1, 2, 5, 6, Sun Pharma, 1, 2, 5, 6, Takeda, 1, 2, 5, 6, UCB, 1, 2, 5, 6; A. Östör: AbbVie, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, GSK, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Janssen, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Lilly, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Novartis, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Pfizer, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials; V. Stakias: AbbVie, 3, 11; T. Madihlaba: AbbVie, 3, 11; R. Lippe: AbbVie, 3, 11; R. Liu: AbbVie, 3, 11; D. Thaçi: AbbVie, 1, 2, 5, 12, Investigator, Almirall, 1, 2, 12, Investigator, Amgen, 1, 2, 12, Investigator, Boehringer-Ingelheim, 1, 2, 12, Investigator, Bristol-Myers Squibb(BMS), 1, 2, 12, Investigator, Celltrion, 1, 2, 12, Investigator, Eli Lilly, 1, 2, 12, Investigator, Galapagos, 1, 2, 12, Investigator, Galderma, 1, 2, 5, 12, Investigator, Janssen-Cilag, 1, 2, 12, Investigator, LEO Pharma, 1, 2, 5, 12, Investigator, Novartis, 1, 2, 5, 12, Investigator, Pfizer, 1, 2, 12, Investigator, Regeneron, 1, 2, 12, Investigator, Samsung, 1, 2, 12, Investigator, Sandoz, 1, 2, 12, Investigator, Sanofi, 1, 2, 12, Investigator, Target-Solution, 1, 2, 12, Investigator, UCB, 1, 2, 12, Investigator.
Background/Purpose: Risankizumab (RZB) is an optimized IL-23 inhibitor (IL-23i) currently approved for the treatment of plaque psoriasis (PsO), PsA, and Crohn's disease. In a post hoc analysis of data from the postmarketing VALUE study in patients with PsO who were treated with RZB or other biologic therapies, we assessed real‑world drug survival for a subgroup of patients also diagnosed with PsA.
Methods: The ongoing, multicountry, prospective observational cohort VALUE study (NCT03982394) enrolled adults (aged ≥ 18 years) who were diagnosed with moderate-to-severe PsO and prescribed RZB or another biologic therapy in accordance with local labeling in a 2:1 ratio. All treatment decisions were made solely by the treating physician. This post hoc analysis included data from the subset of patients with PsO who were also diagnosed with PsA by a rheumatologist and was based on an interim database lock (data cutoff, September 26, 2022). Drug survival was compared for patients receiving RZB vs all other biologics, vs biologics excluding IL-23i, vs TNF-α inhibitors (TNFi), and vs IL-17 inhibitors (IL‑17i). The probability of drug survival was estimated from Kaplan‑Meier curves of time to first drug change (defined as discontinuation of the prescribed biologic treatment or changing to a different biologic treatment). Chi-squared tests were used to evaluate the proportion of patients who had drug changes by month 13. Analyses were repeated after propensity score matching (PSM) with a 1:1 ratio using greedy algorithm and exact match for biologic-naive/biologic-experienced status to account for baseline imbalances between comparison groups.
Results: A total of 244 (RZB) and 189 (other biologics) patients diagnosed with PsO and PsA were included in this analysis. Baseline demographics and characteristics were generally comparable between groups, except patients prescribed RZB vs other biologics were older (median age, 55 vs 51 years, P = .02) and more likely to have prior biologic therapy experience (67.4% vs 43.4%, P < .001). At month 13, fewer patients prescribed RZB had drug changes (5.5%) vs patients prescribed other biologics (19.0%), other biologics excluding IL-23i (20.5%), TNFi (26.7%), and IL‑17i (16.4%; P < .001 for all comparisons). The estimated probability of drug survival [95% CI] at 13 months was higher among patients receiving RZB (95.3% [91.1%, 97.5%]) vs other biologics (80.9% [73.7%, 86.3%]; Figure 1); RZB vs biologics excluding IL-23i (78.9% [70.9%, 85.0%]; Figure 2), or RZB vs TNFi and IL-17i (72.5% [57.3%, 83.1%] and 82.4% [72.0%, 89.2%]; Figure 3). Similar results were observed after PSM (estimated probability of drug survival [95% CI] at 13 months for RZB, 94.8% [88.7%, 97.6%]; other biologics, 84.8% [76.3%, 90.4%]; biologics excluding IL-23i, 82.4% [72.4%, 89.0%]; TNFi, 82.7% [63.2%, 92.4%]; and IL‑17i, 82.8% [69.5%, 90.7%]).
Conclusion: Patients with PsO and PsA who were treated with RZB in the real-world setting experienced fewer drug changes and a higher probability of drug survival compared with those treated with other biologic therapies. These results can help inform clinician decision making when initiating biologic treatment in patients with PsA.



L. Erik: AbbVie, 2, 5, 6, Amgen, 2, 6, Biogen, 2, 6, Bristol-Myers Squibb, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 2, 6, Janssen, 2, 6, MSD, 2, 6, Novartis, 2, 5, 6, Pfizer, 2, 6, UCB, 2, 5, 6; K. Papp: AbbVie, 1, 2, 5, 6, Akros, 1, 2, 5, 6, Amgen, 1, 2, 5, 6, Anacor, 1, 2, 5, 6, Arcutis, 1, 2, 5, 6, Astellas, 1, 2, 5, 6, Bausch Health/Valeant, 1, 2, 5, 6, Baxalta, 1, 2, 5, 6, Boehringer-Ingelheim, 1, 2, 5, 6, Bristol-Myers Squibb, 1, 2, 5, 6, Can-Fite Biopharma, 1, 2, 5, 6, Celgene, 1, 2, 5, 6, Coherus, 1, 2, 5, 6, Dermira, 1, 2, 5, 6, Dow Pharma, 1, 2, 5, 6, Eli Lilly, 1, 2, 5, 6, Evelo, 1, 2, 5, 6, Forward Pharma, 5, Galapagos, 1, 2, 5, 6, Galderma, 1, 2, 5, 6, Genentech, 1, 2, 5, 6, Gilead, 1, 2, 5, 6, GlaxoSmithKlein, 1, 2, 5, 6, Janssen, 1, 2, 5, 6, Kyowa-Hakko Kirin, 1, 2, 5, 6, LEO Pharma, 1, 2, 5, 6, MedImmune, 1, 2, 5, 6, Meiji Seika Pharma, 1, 2, 5, 6, Merck-Serono, 1, 2, 5, 6, Mitsubishi Pharma, 1, 2, 5, 6, Moberg Pharma, 1, 2, 5, 6, MSD, 1, 2, 5, 6, Novartis, 1, 2, 5, 6, Pfizer, 1, 2, 5, 6, PRCL Research, 1, 2, 5, 6, Regeneron, 1, 2, 5, 6, Roche, 1, 2, 5, 6, Sanofi-Aventis/Genzyme, 1, 2, 5, 6, Sun Pharma, 1, 2, 5, 6, Takeda, 1, 2, 5, 6, UCB, 1, 2, 5, 6; A. Östör: AbbVie, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, GSK, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Janssen, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Lilly, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Novartis, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials, Pfizer, 12, has served as a consultant and/or on advisory boards and/or undertaken clinical trials; V. Stakias: AbbVie, 3, 11; T. Madihlaba: AbbVie, 3, 11; R. Lippe: AbbVie, 3, 11; R. Liu: AbbVie, 3, 11; D. Thaçi: AbbVie, 1, 2, 5, 12, Investigator, Almirall, 1, 2, 12, Investigator, Amgen, 1, 2, 12, Investigator, Boehringer-Ingelheim, 1, 2, 12, Investigator, Bristol-Myers Squibb(BMS), 1, 2, 12, Investigator, Celltrion, 1, 2, 12, Investigator, Eli Lilly, 1, 2, 12, Investigator, Galapagos, 1, 2, 12, Investigator, Galderma, 1, 2, 5, 12, Investigator, Janssen-Cilag, 1, 2, 12, Investigator, LEO Pharma, 1, 2, 5, 12, Investigator, Novartis, 1, 2, 5, 12, Investigator, Pfizer, 1, 2, 12, Investigator, Regeneron, 1, 2, 12, Investigator, Samsung, 1, 2, 12, Investigator, Sandoz, 1, 2, 12, Investigator, Sanofi, 1, 2, 12, Investigator, Target-Solution, 1, 2, 12, Investigator, UCB, 1, 2, 12, Investigator.



