Poster Session C
Rheumatoid arthritis (RA)
Session: (2141–2176) RA – Treatment Poster III
2155: The Impact of Filgotinib on Disease Activity Outcomes with Concomitant Pain Control in the Phase 3 FINCH Studies
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- PT
Peter Taylor, PhD, FRCP, MA
University of Oxford
Oxford, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Peter C. Taylor1, Arthur Kavanaugh2, Peter Nash3, Janet Pope4, Georg Pongratz5, Bruno Fautrel6, Rieke Alten7, Ken Hasegawa8, Shangbang Rao9, Dick de Vries10, Pieter-Jan Stiers11, Chris Watson12 and René Westhovens13, 1Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom, 2Division of Rheumatology, Allergy, and Immunology, University of California San Diego, La Jolla, CA, 3School of Medicine, Griffith University, Brisbane, Australia, 4University of Western Ontario, London, ON, Canada, 5Faculty of Medicine, Regensburg University, Regensburg, Germany, Regensburg, Germany, 6Sorbonne Université APHP, Paris, France, 7Department of Internal Medicine and Rheumatology, Schlosspark Klinik, University Medicine Berlin, Berlin, Germany, 8Global Medical Affairs Research, Gilead Sciences, Inc., Foster City, CA, 9Biostatistics, Gilead Sciences, Inc., Foster City, CA, 10Research and Development, Clinical Research, Galapagos BV, Leiden, Netherlands, 11Biostatistics, Galapagos NV, Mechelen, Belgium, 12Medical Affairs, Galapagos Biotech Ltd., Cambridge, United Kingdom, 13Department of Rheumatology, KU Leuven, Skeletal Biology and Engineering Research Center, Leuven, Belgium
Background/Purpose: Patients (pts) with rheumatoid arthritis (RA) often experience substantial pain despite treatment, and consider pain control an important treatment outcome. This post hoc analysis of the FINCH studies assessed specific effects of filgotinib (FIL) on pain and its relationship with efficacy in pts with RA.
Methods: FINCH 1–3 (NCT02889796, NCT02873936, NCT02886728) were Phase 3, randomized, double-blind trials of FIL 100 mg and 200 mg (FIL100/200). In FINCH 1, pts with an inadequate response (IR) to methotrexate (MTX) received FIL, adalimumab (ADA) or placebo (PBO) + MTX for 52 weeks. In FINCH 2, pts with an IR to bDMARDs received FIL or PBO + csDMARDs for 24 weeks. In FINCH 3, MTX-naïve pts received FIL ± MTX or MTX for 52 weeks. For each treatment group, pts reported pain on a 100-mm visual analog scale (VAS). VAS pain ≤ 20 mm indicated health status was not negatively affected by pain; ≤ 10 mm reflected limited to no pain.1 Time to first VAS pain ≤ 10 mm was assessed. The duration of the study period during which VAS pain was ≤ 20 mm or ≤ 10 mm and the proportion of pts who achieved remission (DAS28-CRP < 2.6 or CDAI ≤ 2.8) at Week 24 was evaluated. Of pts who achieved DAS28-CRP or CDAI remission, the proportion who also reported VAS pain ≤ 20 mm or ≤ 10 mm was determined.
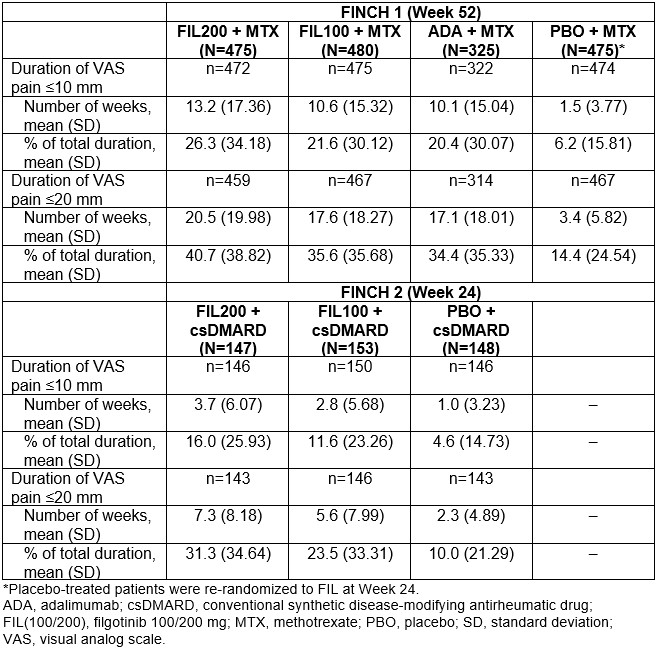
Results: In FINCH 1, there was a higher probability of achieving VAS pain ≤ 10 mm with FIL200, vs ADA + MTX or PBO + MTX; responses were better or comparable with FIL100 vs other treatments. Similar findings were seen in FINCH 2 and 3. In FINCH 1, the time during which VAS pain score was ≤ 20 mm or ≤ 10 mm was greatest in the FIL200 group, and comparable between the FIL100 and ADA groups (Table). The duration of time under each pain threshold was greater in each FIL group vs PBO in FINCH 2 (Table) and in each FIL group vs MTX in FINCH 3. In FINCH 1, the proportion of pts achieving DAS28-CRP remission was greater with FIL200 + MTX (48.4%) and comparable for FIL100 + MTX (35.2%) vs ADA + MTX (35.7%; Figure 1A). The proportion of pts who achieved VAS pain ≤ 20 mm and ≤ 10 mm in addition to DAS28-CRP remission was 35.8% and 26.3%, respectively, in the FIL200 + MTX group, vs 24.6% and 17.2% in the ADA + MTX group (Figure 1A). In FINCH 2, a greater proportion of pts in the FIL groups achieved DAS28-CRP remission and pain responses in addition to DAS28-CRP remission vs the PBO group (Figure 1B). Findings were similar when CDAI remission was assessed for FINCH 1 and 2 (Figure 2A and B). In FINCH 3, the proportion of pts to achieve remission and pain responses in addition to remission was greater in the FIL vs MTX groups; e.g. DAS28-CRP remission and VAS pain ≤ 10 mm was achieved by 23.3% of pts on FIL200, 33.2% on FIL200 + MTX, 27.5% on FIL100 + MTX and 14.9% on MTX.
Conclusion: Across the studies, duration of threshold pain responses achieved over the observation periods was greater with FIL vs comparators. In FINCH 1, FIL200 had a particularly favorable impact when pain response and remission were assessed together; results were comparable between FIL100 and ADA. Similar findings were seen with FIL vs PBO and MTX in FINCH 2 and 3, respectively. These findings suggest that JAK1 inhibition may offer potential added value with respect to patient-reported pain as well as treat-to-target goals.
Reference:
1. Taylor PC, et al. J Clin Med 2019;8:831
.jpg)
.jpg)
P. Taylor: AbbVie, 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 5, Gilead Sciences, 2, GSK, 2, Janssen, 2, Nordic Pharma, 2, Pfizer Inc, 2, Sanofi, 2, UCB, 2; A. Kavanaugh: Amgen, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, Pfizer, 2, UCB, 2; P. Nash: AbbVie, 5, 6, Bristol Myers Squibb, 5, 6, Celgene, 5, 6, Eli Lilly, 5, 6, Galapagos, 5, 6, GSK, 5, 6, Janssen, 5, 6, Novartis, 5, 6, Pfizer Inc, 5, 6; J. Pope: AbbVie, 1, 2; G. Pongratz: AbbVie, 2, 6, Boehringer Ingelheim, 2, 6, Galapagos, 2, Lilly, 2, 6, Pfizer, 2, 6, Roche, 2, 6, Sanofi, 6; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2; R. Alten: AbbVie, 2, 6, Amgen, 2, 6, Biogen, 2, 6, BMS, 2, 6, Celltrion, 2, 6, Gilead, 2, 6, Janssen, 2, 6, Lilly, 2, 6, Medac, 2, 6, MSD, 2, 6, Mylan, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 2, 6, Sandoz, 2, 6, Sanofi-Genzyme, 2, 6, UCB, 2, 6, Viatris, 2, 6; K. Hasegawa: Gilead, 3, 11; S. Rao: Gilead, 3; D. de Vries: Galapagos, 3, 11; P. Stiers: Galapagos, 3, 11; C. Watson: Galapagos, 3, 11; R. Westhovens: Celltrion, 2, 6, Galapagos, 2, 6, Gilead, 2, 6.
Background/Purpose: Patients (pts) with rheumatoid arthritis (RA) often experience substantial pain despite treatment, and consider pain control an important treatment outcome. This post hoc analysis of the FINCH studies assessed specific effects of filgotinib (FIL) on pain and its relationship with efficacy in pts with RA.
Methods: FINCH 1–3 (NCT02889796, NCT02873936, NCT02886728) were Phase 3, randomized, double-blind trials of FIL 100 mg and 200 mg (FIL100/200). In FINCH 1, pts with an inadequate response (IR) to methotrexate (MTX) received FIL, adalimumab (ADA) or placebo (PBO) + MTX for 52 weeks. In FINCH 2, pts with an IR to bDMARDs received FIL or PBO + csDMARDs for 24 weeks. In FINCH 3, MTX-naïve pts received FIL ± MTX or MTX for 52 weeks. For each treatment group, pts reported pain on a 100-mm visual analog scale (VAS). VAS pain ≤ 20 mm indicated health status was not negatively affected by pain; ≤ 10 mm reflected limited to no pain.1 Time to first VAS pain ≤ 10 mm was assessed. The duration of the study period during which VAS pain was ≤ 20 mm or ≤ 10 mm and the proportion of pts who achieved remission (DAS28-CRP < 2.6 or CDAI ≤ 2.8) at Week 24 was evaluated. Of pts who achieved DAS28-CRP or CDAI remission, the proportion who also reported VAS pain ≤ 20 mm or ≤ 10 mm was determined.
Results: In FINCH 1, there was a higher probability of achieving VAS pain ≤ 10 mm with FIL200, vs ADA + MTX or PBO + MTX; responses were better or comparable with FIL100 vs other treatments. Similar findings were seen in FINCH 2 and 3. In FINCH 1, the time during which VAS pain score was ≤ 20 mm or ≤ 10 mm was greatest in the FIL200 group, and comparable between the FIL100 and ADA groups (Table). The duration of time under each pain threshold was greater in each FIL group vs PBO in FINCH 2 (Table) and in each FIL group vs MTX in FINCH 3. In FINCH 1, the proportion of pts achieving DAS28-CRP remission was greater with FIL200 + MTX (48.4%) and comparable for FIL100 + MTX (35.2%) vs ADA + MTX (35.7%; Figure 1A). The proportion of pts who achieved VAS pain ≤ 20 mm and ≤ 10 mm in addition to DAS28-CRP remission was 35.8% and 26.3%, respectively, in the FIL200 + MTX group, vs 24.6% and 17.2% in the ADA + MTX group (Figure 1A). In FINCH 2, a greater proportion of pts in the FIL groups achieved DAS28-CRP remission and pain responses in addition to DAS28-CRP remission vs the PBO group (Figure 1B). Findings were similar when CDAI remission was assessed for FINCH 1 and 2 (Figure 2A and B). In FINCH 3, the proportion of pts to achieve remission and pain responses in addition to remission was greater in the FIL vs MTX groups; e.g. DAS28-CRP remission and VAS pain ≤ 10 mm was achieved by 23.3% of pts on FIL200, 33.2% on FIL200 + MTX, 27.5% on FIL100 + MTX and 14.9% on MTX.
Conclusion: Across the studies, duration of threshold pain responses achieved over the observation periods was greater with FIL vs comparators. In FINCH 1, FIL200 had a particularly favorable impact when pain response and remission were assessed together; results were comparable between FIL100 and ADA. Similar findings were seen with FIL vs PBO and MTX in FINCH 2 and 3, respectively. These findings suggest that JAK1 inhibition may offer potential added value with respect to patient-reported pain as well as treat-to-target goals.
Reference:
1. Taylor PC, et al. J Clin Med 2019;8:831

Table. Duration of threshold pain response achieved over observation period for pain VAS ≤10 mm and ≤20 mm; full analysis set excluding responders at baseline
.jpg)
Figure 1. DAS28-CRP remission and improvement in VAS pain score (up to Week 24) in (A) FINCH 1 and (B) FINCH 2
.jpg)
Figure 2. CDAI remission and improvement in VAS pain score (up to Week 24) in (A) FINCH 1 and (B) FINCH 2
P. Taylor: AbbVie, 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 5, Gilead Sciences, 2, GSK, 2, Janssen, 2, Nordic Pharma, 2, Pfizer Inc, 2, Sanofi, 2, UCB, 2; A. Kavanaugh: Amgen, 2, BMS, 2, Eli Lilly, 2, Novartis, 2, Pfizer, 2, UCB, 2; P. Nash: AbbVie, 5, 6, Bristol Myers Squibb, 5, 6, Celgene, 5, 6, Eli Lilly, 5, 6, Galapagos, 5, 6, GSK, 5, 6, Janssen, 5, 6, Novartis, 5, 6, Pfizer Inc, 5, 6; J. Pope: AbbVie, 1, 2; G. Pongratz: AbbVie, 2, 6, Boehringer Ingelheim, 2, 6, Galapagos, 2, Lilly, 2, 6, Pfizer, 2, 6, Roche, 2, 6, Sanofi, 6; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2; R. Alten: AbbVie, 2, 6, Amgen, 2, 6, Biogen, 2, 6, BMS, 2, 6, Celltrion, 2, 6, Gilead, 2, 6, Janssen, 2, 6, Lilly, 2, 6, Medac, 2, 6, MSD, 2, 6, Mylan, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 2, 6, Sandoz, 2, 6, Sanofi-Genzyme, 2, 6, UCB, 2, 6, Viatris, 2, 6; K. Hasegawa: Gilead, 3, 11; S. Rao: Gilead, 3; D. de Vries: Galapagos, 3, 11; P. Stiers: Galapagos, 3, 11; C. Watson: Galapagos, 3, 11; R. Westhovens: Celltrion, 2, 6, Galapagos, 2, 6, Gilead, 2, 6.



