Poster Session B
Vasculitis
Session: (1554–1578) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
1559: Comparison of Treatment with Adalimumab, Infliximab and Certolizumab in Refractory Cystoid Macular Edema Due to Behçet Disease
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- NB
Nuria Barroso Garcia, -None-
Hospital Universitario Puerta del Mar
Cadiz, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Nuria Barroso-Garcia1, Jose Luis Martin-Varillas2, Lara Sanchez-Bilbao3, Ivan Ferraz Amaro4, Vanesa Calvo Río5, Alfredo Adán6, Inés Hernanz-Rodriguez7, Emma Beltran-Catalan8, David Diaz-Valle9, Marisa Hernandez-Garfella10, Lucia Martinez-Costa11, Manuel Diaz-Llopis12, Jose M Herreras13, Olga Maiz-Alonso14, Ignacio Torre-Salaberri15, Antonio Atanes Sandoval16, Santos Insua-Vilariño17, Raquel Almodovar18, Patricia Fanlo-Mateo19, Juan Ramon De Dios20, Angel Garcia-Aparicio21, Sergio rodriguez-Montero22, Vega Jovani23, Patricia Moya24, Eva Peña Sainz-Pardo25, Jose Luis Hernandez26 and Ricardo Blanco27, 1Hospital Universitario Puerta del Mar, Cadiz, Spain, 2Hospital de Laredo, Laredo, Spain, 3Hospital Universitario Marques de Valdecilla, Santander, Spain, 4Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain, 5Valdecilla Hospital, Santander, Spain, 6Oftalmology, Hospital Clinic de Barcelona, Barcelona, Spain, 7Ophthalmology, Hospital Clinic de Barcelona, Barcelona, Spain, 8HOSPITAL DEL MAR, Barcelona, Spain, 9Hospital Clinico San Carlos, Madrid, Spain, 10Ophthalmology, Hospital Universitario General Valencia, Valencia, Spain, 11Ophthalmology, Hospital Universitario Doctor Peset, Valencia, Spain, 12Ophthalmology, Hospital Universitario La Fe, Valencia, Spain, 13Ophthalmology, Hospital Clínico Universitario de Valladolid, Valladolid, Spain, 14University Hospital Donostia, San Sebastian, Spain, 15Hospital Universitario de Basurto, Bilbao, Spain, 16Rheumatology department, Complexo Hospitalario Universitario A Coruña (CHUAC). Instituto de Investigación Biomédica A Coruña (INIBIC)., A Coruña, Spain, 17Rheumatology, Hospital Universitario de Santiago de Compostela, Santiago Compostela, Spain, 18Alcorcón Foundation University Hospital, Madrid, Spain, 19Internal Medicine, Complejo Hospitalario Universitario de Navarra, Pamplona, Spain, 20Osakidetza, Vitoria, Spain, 21Hospital Universitario de Toledo, Toledo, Spain, 22Rheumatology, Hospital Universitario Virgen de Valme, Sevilla, Spain, 23Department of Rheumatology, Hospital General Universitario Dr. Balmis, Alicante, Spain, 24Hospital de Santa Creu i Sant Pau, Barcelona, Spain, 25Pediatric, Hospital 12 de Octubre, Madrid, Spain, 26Rheumatology, Ophthalmology and Internal Medicine, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 27Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: Cystoid macular edema (CME) is the leading cause of blindness in non-infectious uveitis. One of the most frequently associated conditions is Behçet's disease (BD) (1-3). The objective is to compare efficacy and safety of Adalimumab (ADA), Infliximab (IFX) and Certolizumab (CZP) in CME refractory due to BD.
Methods: Multicenter study of patients with CME secondary to BD refractory to glucocorticoids (GC) and at least 1 conventional immunosuppressant. All patients had CME (OCT >300µ) at baseline. From baseline up to 2 years of follow-up, the evolution of macular thickness (µm), visual acuity (BCVA), anterior chamber (AC) cells, vitritis and GC-sparing effect was analyzed to assess the efficacy of ADA, IFX and CZP. Statistical analysis was performed with IBM SPSS Statistics v.23.
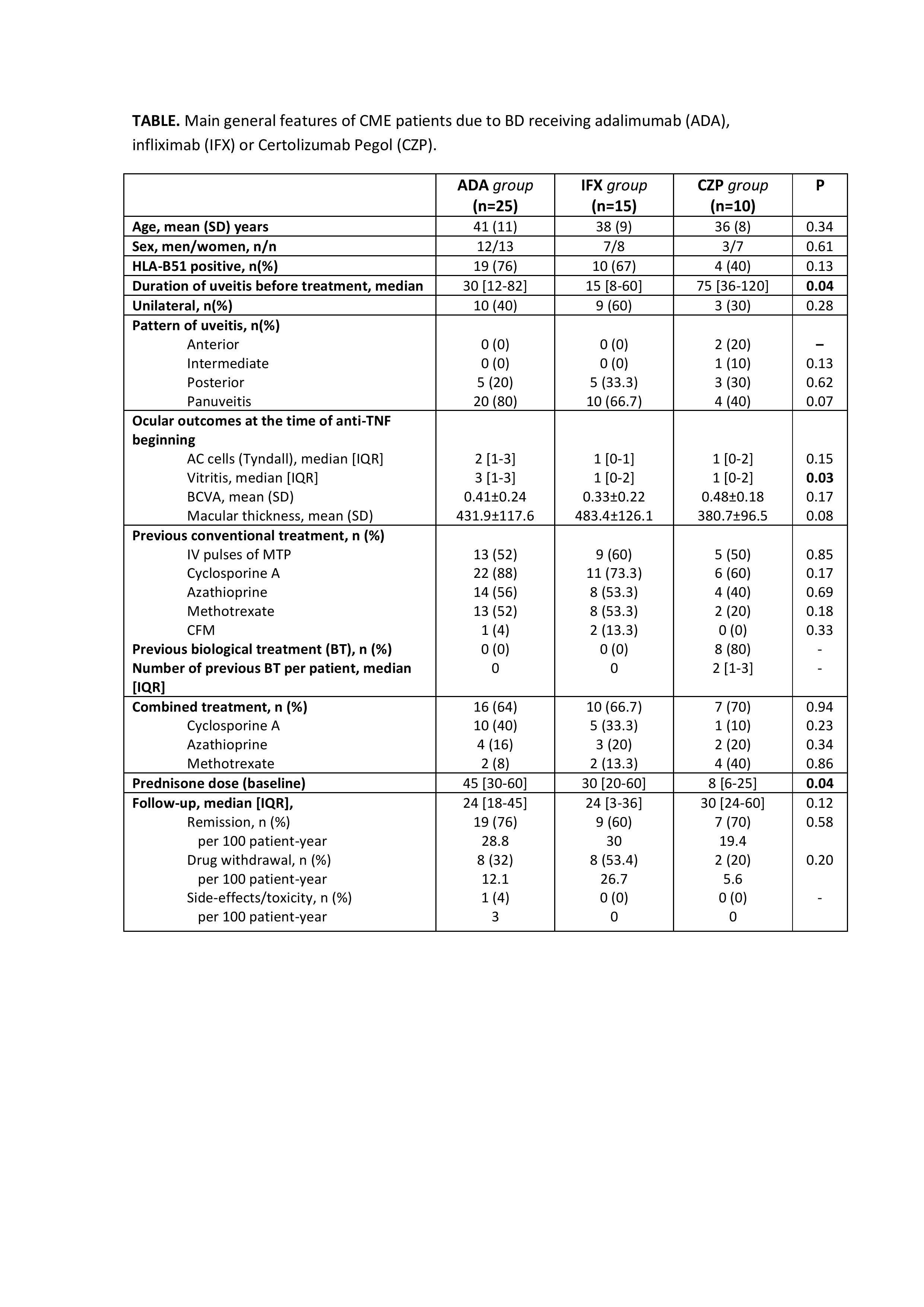
Results: Fifty patients (78 eyes) were evaluated. Twenty-five patients were treated with ADA, 15 with IFX and 10 patients received CZP. No significant differences in demographic parameters were identified in all groups. However, patients in the CZP group had a significantly longer time from diagnosis to drug initiation (75 [36-120] vs 30 [12-82] vs 15 [8-60] months; p=0.04) and had received a greater median [IQR] number of biological treatments (2 [0.75-3] vs 0 [0-0] vs 0 [0-0]) than the ADA and IFX groups. In CZP group, ADA and IFX were used previously in 7 patients. ADA was used in combined therapy in 64%, IFX in 66.7% and CZP in 70% of patients (p=0.94) (TABLE).
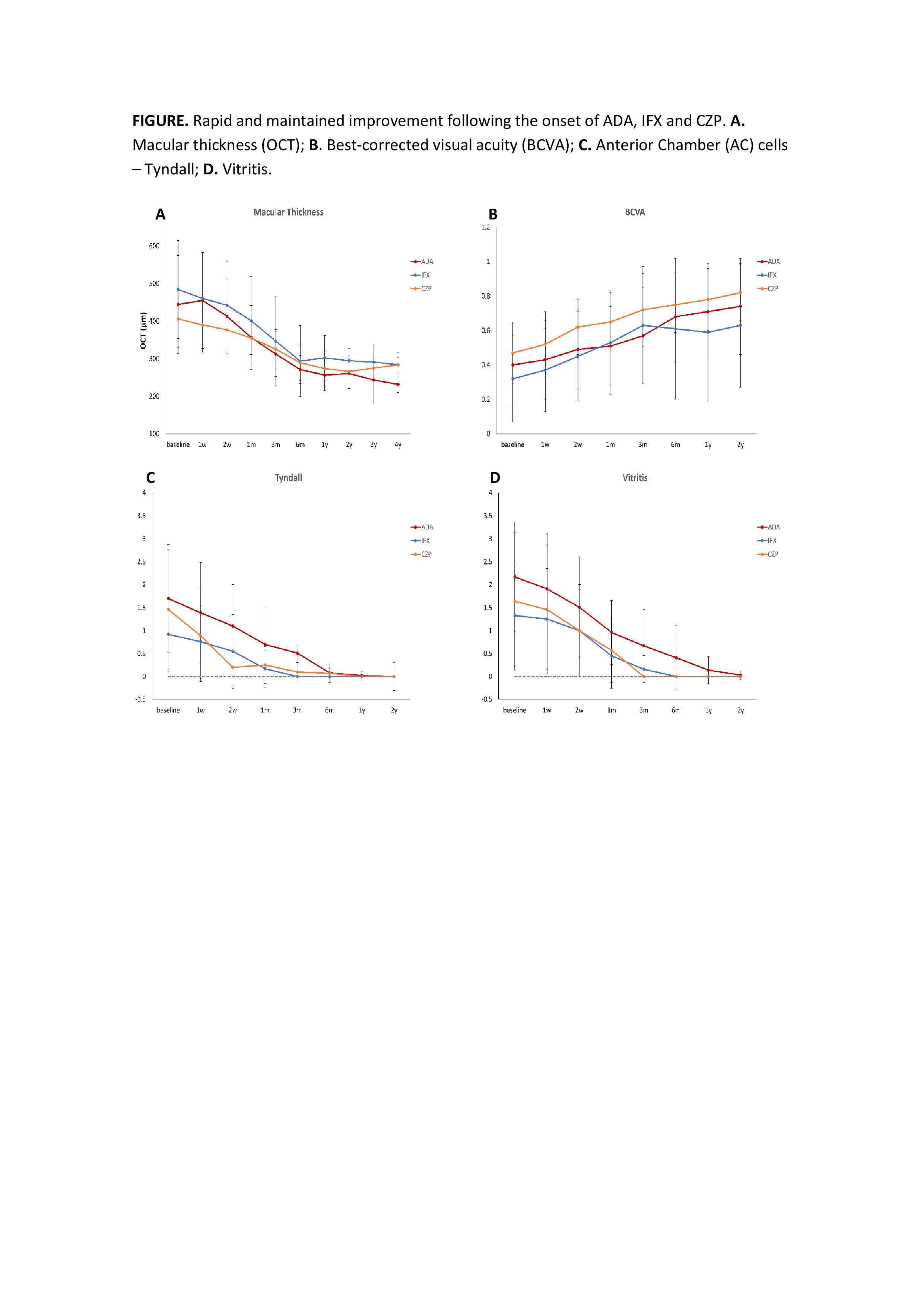
Concerning efficacy outcomes, a rapid and maintained improvement in macular thickness was observed after 2 years of follow-up in three groups with no statistically significant differences between them (FIGURE). Improvement in BCVA, AC cells, vitritis and a GC-sparing effect was also noted. No serious adverse events were observed in IFX and CZP group. One case of pyelonephritis was reported in the ADA group.
Conclusion: ADA, IFX and CZP are effective and safe in refractory CME due to BD. CZP appears effective even in patients with inadequate response to ADA and/or IFX.
N. Barroso-Garcia: None; J. Martin-Varillas: None; L. Sanchez-Bilbao: None; I. Ferraz Amaro: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Bristol-Myers Squibb(BMS), 6; V. Calvo Río: None; A. Adán: None; I. Hernanz-Rodriguez: None; E. Beltran-Catalan: None; D. Diaz-Valle: None; M. Hernandez-Garfella: None; L. Martinez-Costa: None; M. Diaz-Llopis: None; J. Herreras: None; O. Maiz-Alonso: None; I. Torre-Salaberri: None; A. Atanes Sandoval: None; S. Insua-Vilariño: None; R. Almodovar: None; P. Fanlo-Mateo: None; J. De Dios: None; A. Garcia-Aparicio: None; S. rodriguez-Montero: None; V. Jovani: None; P. Moya: None; E. Peña Sainz-Pardo: None; J. Hernandez: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: Cystoid macular edema (CME) is the leading cause of blindness in non-infectious uveitis. One of the most frequently associated conditions is Behçet's disease (BD) (1-3). The objective is to compare efficacy and safety of Adalimumab (ADA), Infliximab (IFX) and Certolizumab (CZP) in CME refractory due to BD.
Methods: Multicenter study of patients with CME secondary to BD refractory to glucocorticoids (GC) and at least 1 conventional immunosuppressant. All patients had CME (OCT >300µ) at baseline. From baseline up to 2 years of follow-up, the evolution of macular thickness (µm), visual acuity (BCVA), anterior chamber (AC) cells, vitritis and GC-sparing effect was analyzed to assess the efficacy of ADA, IFX and CZP. Statistical analysis was performed with IBM SPSS Statistics v.23.
Results: Fifty patients (78 eyes) were evaluated. Twenty-five patients were treated with ADA, 15 with IFX and 10 patients received CZP. No significant differences in demographic parameters were identified in all groups. However, patients in the CZP group had a significantly longer time from diagnosis to drug initiation (75 [36-120] vs 30 [12-82] vs 15 [8-60] months; p=0.04) and had received a greater median [IQR] number of biological treatments (2 [0.75-3] vs 0 [0-0] vs 0 [0-0]) than the ADA and IFX groups. In CZP group, ADA and IFX were used previously in 7 patients. ADA was used in combined therapy in 64%, IFX in 66.7% and CZP in 70% of patients (p=0.94) (TABLE).
Concerning efficacy outcomes, a rapid and maintained improvement in macular thickness was observed after 2 years of follow-up in three groups with no statistically significant differences between them (FIGURE). Improvement in BCVA, AC cells, vitritis and a GC-sparing effect was also noted. No serious adverse events were observed in IFX and CZP group. One case of pyelonephritis was reported in the ADA group.
Conclusion: ADA, IFX and CZP are effective and safe in refractory CME due to BD. CZP appears effective even in patients with inadequate response to ADA and/or IFX.


N. Barroso-Garcia: None; J. Martin-Varillas: None; L. Sanchez-Bilbao: None; I. Ferraz Amaro: AbbVie/Abbott, 5, 6, Amgen, 5, 6, Bristol-Myers Squibb(BMS), 6; V. Calvo Río: None; A. Adán: None; I. Hernanz-Rodriguez: None; E. Beltran-Catalan: None; D. Diaz-Valle: None; M. Hernandez-Garfella: None; L. Martinez-Costa: None; M. Diaz-Llopis: None; J. Herreras: None; O. Maiz-Alonso: None; I. Torre-Salaberri: None; A. Atanes Sandoval: None; S. Insua-Vilariño: None; R. Almodovar: None; P. Fanlo-Mateo: None; J. De Dios: None; A. Garcia-Aparicio: None; S. rodriguez-Montero: None; V. Jovani: None; P. Moya: None; E. Peña Sainz-Pardo: None; J. Hernandez: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



