Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (2352–2369) Systemic Sclerosis & Related Disorders – Clinical Poster III: Translational Science
2362: Identification of Protein Biomarkers Associated with the Severity and Risk of Progression of Interstitial Lung Disease in VEDOSS and Established Systemic Sclerosis Patients
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- FD
Francesco Del Galdo, MD, PhD (he/him/his)
University of Leeds
Leeds, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Thierry Sornasse1, Vishal Kakkar2, Rebecca Ross3, Sunhwa Kim4 and Francesco Del Galdo5, 1AbbVie, Inc., North Chicago, IL, 2University of Leeds, Leeds, United Kingdom, 3Medicine and Health, University of Leeds, Leeds, United Kingdom, 4AbbVie, Inc., South San Francisco, CA, 5University of Leeds - Leeds Institute of Rheumatic and Muskuloskeletal Medicine, Leeds, United Kingdom
Background/Purpose: Pulmonary Fibrosis (PF) is one of the primary causes of systemic sclerosis (SSc)-related death. Hence, monitoring and predicting the course of PF in SSc remains a critical need in patient management. Furthermore, the stratification of patients based on their risk of PF progression should be improved the feasibility of clinical studies. Here, we explore the relationships of a subset of protein biomarkers (pBMs), initially identified as prognostic of disease worsening in progressing fibrosing interstitial lung disease (ILD) (Bowman WS et al Lancet Respir Medicine 2022), with the severity and risk of PF in Very Early Diagnosis of SSc (VEDOSS) and established limited (lcSSc) and diffuse cutaneous SSc (dcSSc).
Methods: Hundreds one patients (33 VEDOSS, 21 lcSSc, and 47 dcSSc) were selected from the ongoing SSc registry at the University of Leeds. Using a proximity extension assay, we determined the relative levels of 34 PF-ILD-related pBMs in the serum samples. Association with the presence of ILD at baseline was calculated using a t-test, with the severity of ILD (Percent Predicted FVC) at baseline using Pearson's correlation, and with the risk of FVC decline using a Proportional Hazard model based on the median-dichotomized pBM levels at baseline. All 101 patients were combined for these analyses, and Benjamini-Hochberg FDR correction for multiple comparisons was applied.
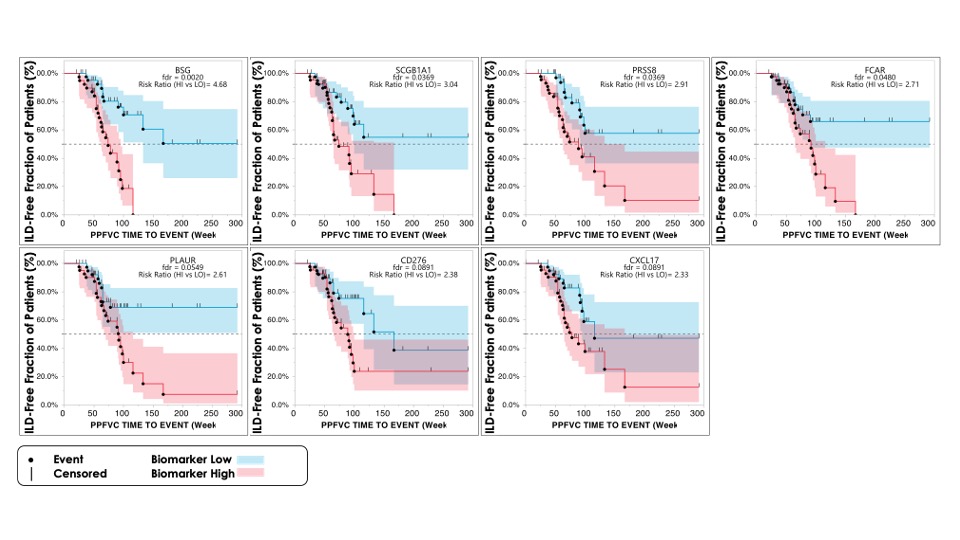
Results: Out of 34 pBMs, we identified 8 BMs differentially expressed between VEDOSS, lcSSc, and dcSSc patients with or without ILD at baseline. AGER was lower in patients with ILD, suggesting a positive beneficial relation with ILD. Conversely, the remaining 7 pBMs (ITGB6, KRT19, PRSS8, SCGB3A2, IL1RN, SERPINB8, and HGF) were higher in patients with ILD, suggesting a potentially detrimental link with ILD. Consistent with these observations, AGER correlated positively with baseline ppFVC, while the other 5 pBMs correlated negatively with baseline ppFVC. Finally, we identified 7 pBMs (BSG, SCGB1A1, PLAUR, FCAR, PRSS8, CD276, and CXCL17) associated with the relative risk of ILD progression (figure 1; as defined by a reduction of ppFVC of 0.05/week). PRSS8 was the only pBM shared across the three analyses, suggesting a possible role of this peptidase in lung fibrosis.
Conclusion: In this pilot study, we confirmed the association of a subset of the pBMs identified by Bowman et al. with the presence, severity, and risks of progression of ILD in VEDOSS, lcSSc, and dcSSc patients. Notably, the pBMs associated with the presence and severity of lung fibrosis differed from those associated with the risk of progression. While these results could lead to useful biomarker tools for the stratification and monitoring of lung fibrosis in VEDOSS and SSc patients, we need to confirm these results in an additional group of prospectively recruited VEDOSS and established SSc.
T. Sornasse: AbbVie, 3, 11; V. Kakkar: None; R. Ross: None; S. Kim: AbbVie/Abbott, 3, Merck/MSD, 11; F. Del Galdo: AbbVie/Abbott, 5, arxx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, capella, 2, Chemomab, 2, GlaxoSmithKlein(GSK), 2, Janssen, 2, Mitsubishi-Tanabe, 2, 5.
Background/Purpose: Pulmonary Fibrosis (PF) is one of the primary causes of systemic sclerosis (SSc)-related death. Hence, monitoring and predicting the course of PF in SSc remains a critical need in patient management. Furthermore, the stratification of patients based on their risk of PF progression should be improved the feasibility of clinical studies. Here, we explore the relationships of a subset of protein biomarkers (pBMs), initially identified as prognostic of disease worsening in progressing fibrosing interstitial lung disease (ILD) (Bowman WS et al Lancet Respir Medicine 2022), with the severity and risk of PF in Very Early Diagnosis of SSc (VEDOSS) and established limited (lcSSc) and diffuse cutaneous SSc (dcSSc).
Methods: Hundreds one patients (33 VEDOSS, 21 lcSSc, and 47 dcSSc) were selected from the ongoing SSc registry at the University of Leeds. Using a proximity extension assay, we determined the relative levels of 34 PF-ILD-related pBMs in the serum samples. Association with the presence of ILD at baseline was calculated using a t-test, with the severity of ILD (Percent Predicted FVC) at baseline using Pearson's correlation, and with the risk of FVC decline using a Proportional Hazard model based on the median-dichotomized pBM levels at baseline. All 101 patients were combined for these analyses, and Benjamini-Hochberg FDR correction for multiple comparisons was applied.
Results: Out of 34 pBMs, we identified 8 BMs differentially expressed between VEDOSS, lcSSc, and dcSSc patients with or without ILD at baseline. AGER was lower in patients with ILD, suggesting a positive beneficial relation with ILD. Conversely, the remaining 7 pBMs (ITGB6, KRT19, PRSS8, SCGB3A2, IL1RN, SERPINB8, and HGF) were higher in patients with ILD, suggesting a potentially detrimental link with ILD. Consistent with these observations, AGER correlated positively with baseline ppFVC, while the other 5 pBMs correlated negatively with baseline ppFVC. Finally, we identified 7 pBMs (BSG, SCGB1A1, PLAUR, FCAR, PRSS8, CD276, and CXCL17) associated with the relative risk of ILD progression (figure 1; as defined by a reduction of ppFVC of 0.05/week). PRSS8 was the only pBM shared across the three analyses, suggesting a possible role of this peptidase in lung fibrosis.
Conclusion: In this pilot study, we confirmed the association of a subset of the pBMs identified by Bowman et al. with the presence, severity, and risks of progression of ILD in VEDOSS, lcSSc, and dcSSc patients. Notably, the pBMs associated with the presence and severity of lung fibrosis differed from those associated with the risk of progression. While these results could lead to useful biomarker tools for the stratification and monitoring of lung fibrosis in VEDOSS and SSc patients, we need to confirm these results in an additional group of prospectively recruited VEDOSS and established SSc.

T. Sornasse: AbbVie, 3, 11; V. Kakkar: None; R. Ross: None; S. Kim: AbbVie/Abbott, 3, Merck/MSD, 11; F. Del Galdo: AbbVie/Abbott, 5, arxx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, capella, 2, Chemomab, 2, GlaxoSmithKlein(GSK), 2, Janssen, 2, Mitsubishi-Tanabe, 2, 5.



