Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
2266: Direct Health Care Costs Differ by SLE Autoantibody Machine Learning Clusters in an International Inception
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MC
May Choi, MD, FRCPC, MPH
University of Calgary
Calgary, AB, CanadaDisclosure(s): No relevant disclosure to display
Abstract Poster Presenter(s)
May Choi1, Karen Costenbader2, Marvin Fritzler1, Yvan St. Pierre3, Murray Urowitz4, John G. Hanly5, Caroline Gordon6, Sang-Cheol Bae7, Juanita Romero-Diaz8, Jorge Sanchez-Guerrero9, Sasha Bernatsky3, Daniel Wallace10, David Isenberg11, Anisur Rahman12, Joan Merrill13, Paul R. Fortin14, Dafna Gladman15, Ian Bruce16, Michelle Petri17, Ellen Ginzler18, Mary Anne Dooley19, Rosalind Ramsey-Goldman20, Susan Manzi21, Andreas Jonsen22, Graciela S Alarcón23, Ronald van Vollenhoven24, Cynthia Aranow25, Meggan MacKay25, Guillermo Ruiz-Irastorza26, S. Sam Lim27, Murat Inanc28, Kenneth Kalunian29, Soren Jacobsen30, Christine Peschken31, Diane L. Kamen32, Anca Askanase33, Jill Buyon34 and Ann Clarke35, 1University of Calgary, Calgary, AB, Canada, 2Brigham and Women's Hospital and Harvard Medical School, Boston, MA, 3Research Institute of the McGill University Health Centre, Montreal, QC, Canada, 4Schroeder Arthritis Institute, Krembil Research Institute; University of Toronto Lupus Clinic; Division of Rheumatology, Toronto, ON, Canada, 5Dalhousie University, Halifax, NS, Canada, 6Institute of Inflammation and Ageing, University of Birmingham, Birmingham, United Kingdom, 7Hanyang University Hospital for Rheumatic Diseases and Hanyang University Institute for Rheumatology Research, Department of Rheumatology, Seoul, South Korea, 8Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico, 9University Health Network, Toronto, ON, Canada, 10Cedars-Sinai Medical Center, Los Angeles, CA, 11University College London, London, United Kingdom, 12Centre for Rheumatology, Division of Medicine, University College London, London, United Kingdom, 13Oklahoma Medical Research Foundation, Oklahoma City, OK, 14Centre ARThrite - CHU de Québec - Université Laval, Quebec City, QC, Canada, 15Schroeder Arthritis Institute, Krembil Research Institute, Toronto Western Hospital, Department of Medicine, University of Toronto, Toronto, ON, Canada, 16University of Manchester, Manchester, United Kingdom, 17Department of Medicine, Division of Rheumatology, Johns Hopkins University School of Medicine, Timonium, MD, 18SUNY Downstate Health Sciences University, Brooklyn, NY, 19Raleigh Neurology Associates, Chapel Hill, NC, 20Northwestern University, Chicago, IL, 21Lupus Center of Excellence, Autoimmunity Institute, Allegheny Health Network, Pittsburgh, PA, 22Rheumatology, Department of Clinical Sciences, Lund University, Lund, Sweden, 23Heersink School of Medicine. The University of Alabama at Birmingham, Birmingham, AL, 24Amsterdam University Medical Centers, Amsterdam, Netherlands, 25Feinstein Institutes for Medical Research, Manhasset, NY, 26Hospital Universitario Cruces, Barakaldo, Spain, 27Emory University, Atlanta, GA, 28Division of Rheumatology, Department of Internal Medicine, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey, 29University of California San Diego, La Jolla, CA, 30Rigshospitalet, Copenhagen, Denmark, 31University of Manitoba, Winnipeg, MB, Canada, 32Medical University of South Carolina, Charleston, SC, 33Columbia University Medical Center, New York, NY, 34NYU Grossman School of Medicine, New York, NY, 35University of Calgary, Division of Rheumatology, Cumming School of Medicine, Calgary, AB, Canada
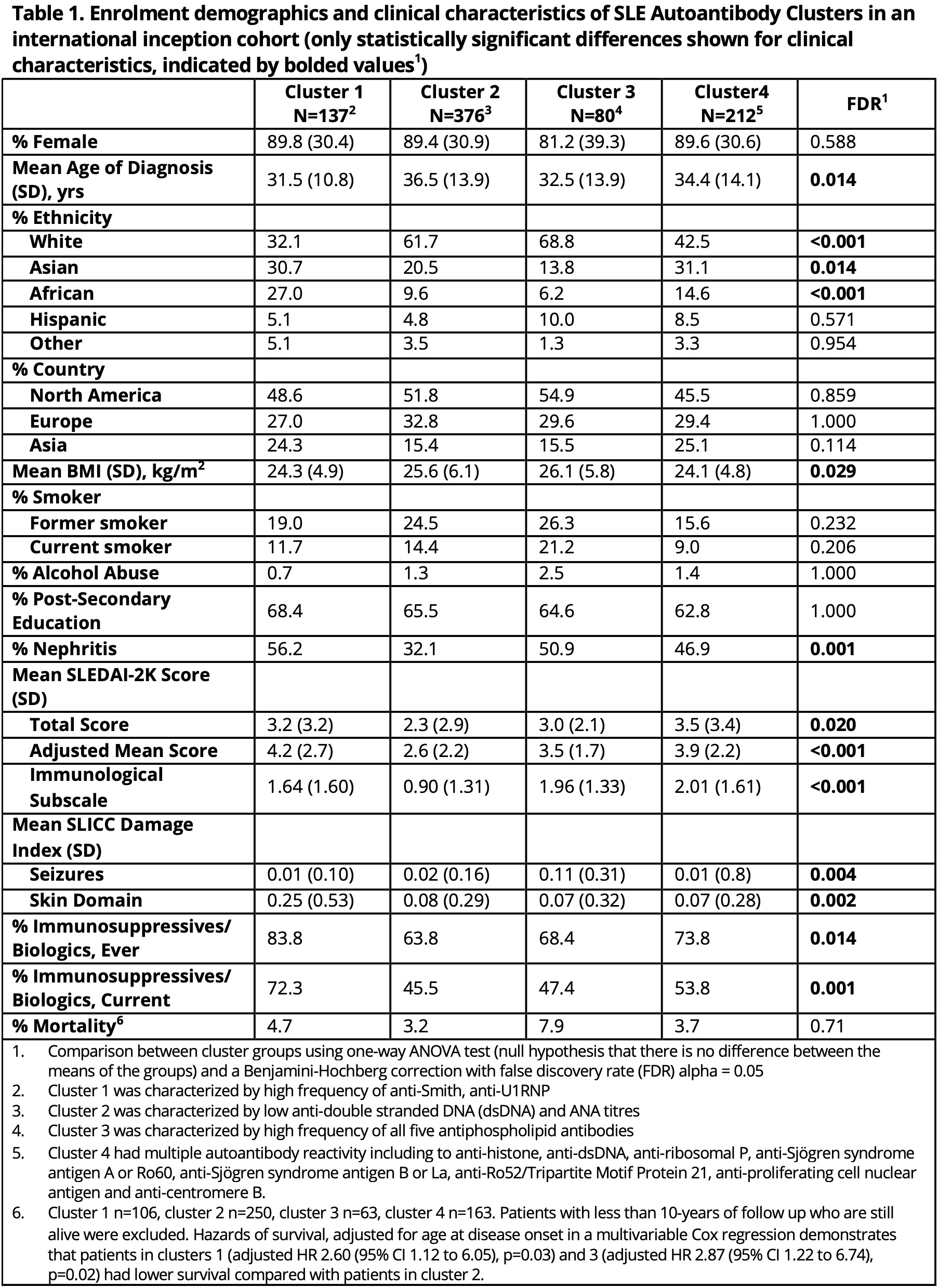
Background/Purpose: Using machine learning, we identified 4 patient clusters based on longitudinal autoantibody profiles in an international SLE inception cohort, which were predictive of disease outcomes (Ann Rheum Dis 2023). We now compare direct and indirect costs between these SLE clusters to elucidate healthcare utilization patterns in SLE.
Methods: Patients fulfilling the 1997 Revised ACR SLE Classification Criteria from 33 centres (11 countries) were enrolled within 15 months of diagnosis and clustered by k-means using longitudinal 29 ANA immunofluorescence pattern and 20 autoantibody profiles. Data were collected annually on health care use (i.e., hospitalizations, medications, dialysis, and selected procedures, as well as SLE antibodies, organ involvement, activity [adjusted mean SLEDAI-2K] and medication use), supplemented by data on additional resource use and lost work-force/non-work-force productivity in a patient subset. Multiple imputation was used to predict all missing values for the patients in the full cohort who did not provide direct/indirect costs for all observations. Health care use was costed using 2023 Canadian prices and lost productivity using Statistics Canada age-and-sex specific wages. Average annual costs over follow up were compared between clusters using multivariable regressions, adjusting for significant predictors for direct and indirect costs.
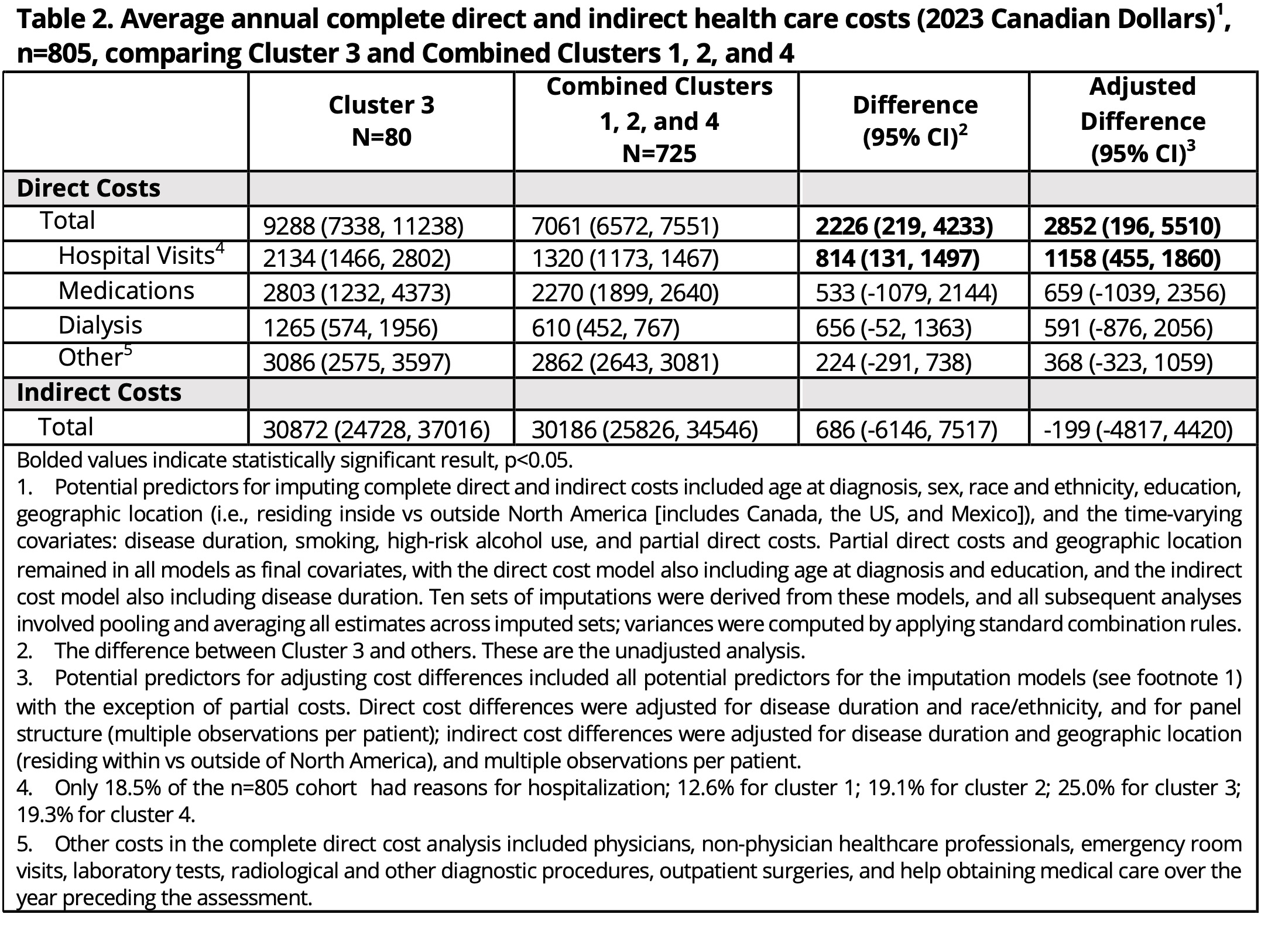
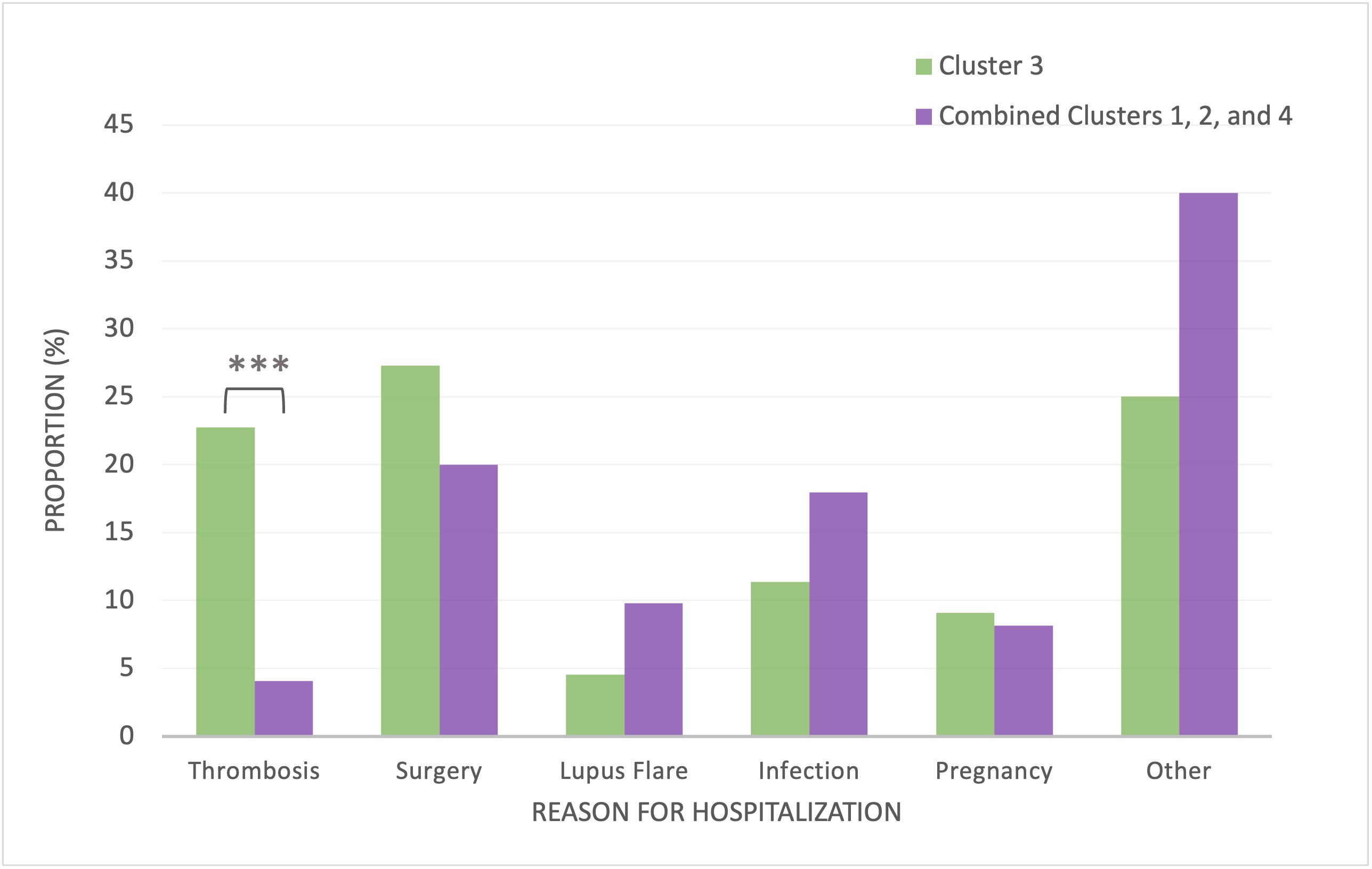
Results: Of 1800+ patients enrolled in the SLICC cohort, 805 subjects were included in the SLE clusters and provided cost data (Table 1). Mean follow-up time for the entire cohort was 12.3 years (range 2.9-21.6 years) and similar across clusters. There were no clear differences in direct and indirect costs and component costs between clusters 1 (high frequency of anti-Sm/anti-U1RNP, predictive of high cumulative disease activity and immunosuppressant/biologic use), 2 (low autoantibody reactivity, predictive of low disease activity and immunosuppressant/biologic use), and 4 (multiple autoantibody reactivities, predictive of high disease activity). Thus, these 3 clusters were combined and compared with cluster 3 (highest frequency of all antiphospholipid antibodies [IgM/IgG anti-cardiolipin, -ß2GP1, -PS/PT, IgG anti-ß2GP1D1, lupus anticoagulant], predictive of seizures and mortality) using multivariable regressions. Cluster 3 had higher total direct costs than clusters 1, 2, and 4 combined ($9288 vs $7061; adj. diff. $2852 [95%CI $196, $5510]), particularly for hospitalizations ($2134 vs $1320; adj. diff. $1158 [95%CI $455, $1860]) (Table 2). Cluster 3 had significantly more hospitalizations for thrombotic/cardiovascular (CVD) events compared to combined clusters (2-sample test of proportions, 22.7% vs. 4.1%, diff. 18.6% [95%CI 6.0%, 31.3%]) (Fig1).
Conclusion: Machine learning based on autoantibodies alone identified a patient cluster with high antiphospholipid antibody frequency who incurred the greatest direct costs, a high proportion driven by hospitalizations due to thrombosis/CVD-related events. Even clusters with severe SLE did not incur such high cost, suggesting thrombotic and antiphospholipid-related complications are important contributors to the economic burden of SLE.
M. Choi: AbbVie/Abbott, 2, 6, Amgen, 2, 6, AstraZeneca, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 6, Mallinckrodt, 2, Merck/MSD, 2, MitogenDx, 2, Organon, 6, Pfizer, 2, 6, Roche, 2, Werfen, 2; K. Costenbader: Amgen, 2, 5, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 2, Cabaletta, 2, Eli Lilly, 2, Exagen Diagnostics, 5, Gilead, 5, GlaxoSmithKlein(GSK), 2, 5, Janssen, 2, 5; M. Fritzler: None; Y. St. Pierre: None; M. Urowitz: None; J. Hanly: None; C. Gordon: AbbVie, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, Sanofi, 2, UCB Pharma, 2; S. Bae: None; J. Romero-Diaz: None; J. Sanchez-Guerrero: None; S. Bernatsky: None; D. Wallace: None; D. Isenberg: None; A. Rahman: None; J. Merrill: AbbVie, 2, Alexion, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, 5, Aurinia, 2, Bristol Myers Squibb, 2, 5, EMD Serono, 2, Genentech, 2, Gilead, 2, GlaxoSmithKline, 2, 5, Lilly, 2, Merck, 2, Pfizer, 2, Provention, 2, Remegen, 2, Sanofi, 2, UCB Pharma, 2, Zenas, 2; P. Fortin: AbbVie, 1, AstraZeneca, 1, 6, GlaxoSmithKlein(GSK), 1, 6, Roche-Genentech, 1; D. Gladman: AbbVie, 2, 5, Amgen, 2, 5, Bristol Myers Squibb, 2, Celgene, 2, 5, Eli Lilly, 2, 5, Galapagos, 2, Gilead Sciences, 2, Janssen, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, UCB, 2, 5; I. Bruce: AstraZeneca, 1, 2, 5, 6, Aurinia, 2, GSK, 1, 2, 5, 6, Janssen, 5, 6, Lilly, 1, UCB, 6; M. Petri: Alexion, 1, Amgen, 1, AnaptysBio, 1, Annexon Bio, 1, Argenx, 1, Arhros-Focus Med/Ed, 6, AstraZeneca, 1, 5, Aurinia, 1, 5, 6, Axdev, 1, Biogen, 1, Boxer Capital, 2, Cabaletto Bio, 2, Caribou Biosciences, 2, CVS Health, 1, Eli Lilly, 1, 5, Emergent Biosolutions, 1, Exagen, 5, Exo Therapeutics, 2, Gilead Biosciences, 2, GlaxoSmithKlein(GSK), 1, 5, 6, Horizon Therapeutics, 2, Idorsia Pharmaceuticals, 2, IQVIA, 1, Janssen, 1, 5, Kira Pharmaceuticals, 2, MedShr, 6, Merck/EMD Serono, 1, Momenta Pharmaceuticals, 2, Nexstone Immunology, 2, Nimbus Lakshmi, 2, Proviant, 2, Sanofi, 2, Sinomab Biosciences, 2, Thermofisher, 5, UCB, 2; E. Ginzler: None; M. Dooley: None; R. Ramsey-Goldman: Ampel Solutions, 2, Calabetta, 2, Exagen, 2, Immunocor, 6; S. Manzi: AbbVie, 5, Allegheny Singer Research Institute, 10, AstraZeneca, 2, 5, Exagen Diagnostics, Inc, 2, 9, 10, GSK, 2, 5, Lilly, 2, Lupus Foundation of America, 4, Novartis, 2, UCB Advisory Board, 2, Univiersity of Pittsburgh, 10; A. Jonsen: None; G. Alarcón: None; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 5, 6, Biogen, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 5, 6, GlaxoSmithKline, 6, Janssen, 2, 6, MSD/Merck Sharp and Dohme, 5, Novartis, 5, Pfizer, 2, 5, 6, RemeGen, 2, Roche, 5, Sanofi, 5, UCB, 2, 5, 6; C. Aranow: AstraZeneca, 2, Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, 5, kezar Inc, 2; M. MacKay: None; G. Ruiz-Irastorza: None; S. Lim: None; M. Inanc: None; K. Kalunian: AbbVie/Abbott, 2, Amgen, 5, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb(BMS), 2, Eli Lilly, 2, EquilliumBio, 2, Genentech, 2, Gilead, 2, Janssen, 2, KezarBio, 1, Merck/MSD, 2, Novartis, 2, Pfizer, 2, Remegene, 2, Roche, 2, UCB, 5; S. Jacobsen: None; C. Peschken: AstraZeneca, 2, 5, GSK, 2, 5, Roche, 1, 2; D. Kamen: None; A. Askanase: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb, 2, Celgene, 2, Eli Lilly, 2, Genentech, 2, GSK, 2, Idorsia, 2, Janssen, 2, Mallinckrodt, 2, Pfizer, 2, UCB Pharma, 2; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; A. Clarke: AstraZeneca, 2, Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, 5, Otsuka, 2, Roche, 2.
Background/Purpose: Using machine learning, we identified 4 patient clusters based on longitudinal autoantibody profiles in an international SLE inception cohort, which were predictive of disease outcomes (Ann Rheum Dis 2023). We now compare direct and indirect costs between these SLE clusters to elucidate healthcare utilization patterns in SLE.
Methods: Patients fulfilling the 1997 Revised ACR SLE Classification Criteria from 33 centres (11 countries) were enrolled within 15 months of diagnosis and clustered by k-means using longitudinal 29 ANA immunofluorescence pattern and 20 autoantibody profiles. Data were collected annually on health care use (i.e., hospitalizations, medications, dialysis, and selected procedures, as well as SLE antibodies, organ involvement, activity [adjusted mean SLEDAI-2K] and medication use), supplemented by data on additional resource use and lost work-force/non-work-force productivity in a patient subset. Multiple imputation was used to predict all missing values for the patients in the full cohort who did not provide direct/indirect costs for all observations. Health care use was costed using 2023 Canadian prices and lost productivity using Statistics Canada age-and-sex specific wages. Average annual costs over follow up were compared between clusters using multivariable regressions, adjusting for significant predictors for direct and indirect costs.
Results: Of 1800+ patients enrolled in the SLICC cohort, 805 subjects were included in the SLE clusters and provided cost data (Table 1). Mean follow-up time for the entire cohort was 12.3 years (range 2.9-21.6 years) and similar across clusters. There were no clear differences in direct and indirect costs and component costs between clusters 1 (high frequency of anti-Sm/anti-U1RNP, predictive of high cumulative disease activity and immunosuppressant/biologic use), 2 (low autoantibody reactivity, predictive of low disease activity and immunosuppressant/biologic use), and 4 (multiple autoantibody reactivities, predictive of high disease activity). Thus, these 3 clusters were combined and compared with cluster 3 (highest frequency of all antiphospholipid antibodies [IgM/IgG anti-cardiolipin, -ß2GP1, -PS/PT, IgG anti-ß2GP1D1, lupus anticoagulant], predictive of seizures and mortality) using multivariable regressions. Cluster 3 had higher total direct costs than clusters 1, 2, and 4 combined ($9288 vs $7061; adj. diff. $2852 [95%CI $196, $5510]), particularly for hospitalizations ($2134 vs $1320; adj. diff. $1158 [95%CI $455, $1860]) (Table 2). Cluster 3 had significantly more hospitalizations for thrombotic/cardiovascular (CVD) events compared to combined clusters (2-sample test of proportions, 22.7% vs. 4.1%, diff. 18.6% [95%CI 6.0%, 31.3%]) (Fig1).
Conclusion: Machine learning based on autoantibodies alone identified a patient cluster with high antiphospholipid antibody frequency who incurred the greatest direct costs, a high proportion driven by hospitalizations due to thrombosis/CVD-related events. Even clusters with severe SLE did not incur such high cost, suggesting thrombotic and antiphospholipid-related complications are important contributors to the economic burden of SLE.



Figure 1. Reasons for Hospitalization Cluster 3 vs. Combined Clusters 1, 2, and 4. There was a higher proportion of hospitalizations for thrombotic events (e.g., myocardial infarction, stroke, pulmonary embolism) for Cluster 3 (*** p<0.001) compared to Combined Clusters 1, 2, and 4.
M. Choi: AbbVie/Abbott, 2, 6, Amgen, 2, 6, AstraZeneca, 2, 6, Bristol-Myers Squibb(BMS), 2, 6, Celgene, 2, 6, Eli Lilly, 2, 6, GlaxoSmithKlein(GSK), 2, Janssen, 2, 6, Mallinckrodt, 2, Merck/MSD, 2, MitogenDx, 2, Organon, 6, Pfizer, 2, 6, Roche, 2, Werfen, 2; K. Costenbader: Amgen, 2, 5, AstraZeneca, 5, Bristol-Myers Squibb(BMS), 2, Cabaletta, 2, Eli Lilly, 2, Exagen Diagnostics, 5, Gilead, 5, GlaxoSmithKlein(GSK), 2, 5, Janssen, 2, 5; M. Fritzler: None; Y. St. Pierre: None; M. Urowitz: None; J. Hanly: None; C. Gordon: AbbVie, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, Sanofi, 2, UCB Pharma, 2; S. Bae: None; J. Romero-Diaz: None; J. Sanchez-Guerrero: None; S. Bernatsky: None; D. Wallace: None; D. Isenberg: None; A. Rahman: None; J. Merrill: AbbVie, 2, Alexion, 2, Alumis, 2, Amgen, 2, AstraZeneca, 2, 5, Aurinia, 2, Bristol Myers Squibb, 2, 5, EMD Serono, 2, Genentech, 2, Gilead, 2, GlaxoSmithKline, 2, 5, Lilly, 2, Merck, 2, Pfizer, 2, Provention, 2, Remegen, 2, Sanofi, 2, UCB Pharma, 2, Zenas, 2; P. Fortin: AbbVie, 1, AstraZeneca, 1, 6, GlaxoSmithKlein(GSK), 1, 6, Roche-Genentech, 1; D. Gladman: AbbVie, 2, 5, Amgen, 2, 5, Bristol Myers Squibb, 2, Celgene, 2, 5, Eli Lilly, 2, 5, Galapagos, 2, Gilead Sciences, 2, Janssen, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, UCB, 2, 5; I. Bruce: AstraZeneca, 1, 2, 5, 6, Aurinia, 2, GSK, 1, 2, 5, 6, Janssen, 5, 6, Lilly, 1, UCB, 6; M. Petri: Alexion, 1, Amgen, 1, AnaptysBio, 1, Annexon Bio, 1, Argenx, 1, Arhros-Focus Med/Ed, 6, AstraZeneca, 1, 5, Aurinia, 1, 5, 6, Axdev, 1, Biogen, 1, Boxer Capital, 2, Cabaletto Bio, 2, Caribou Biosciences, 2, CVS Health, 1, Eli Lilly, 1, 5, Emergent Biosolutions, 1, Exagen, 5, Exo Therapeutics, 2, Gilead Biosciences, 2, GlaxoSmithKlein(GSK), 1, 5, 6, Horizon Therapeutics, 2, Idorsia Pharmaceuticals, 2, IQVIA, 1, Janssen, 1, 5, Kira Pharmaceuticals, 2, MedShr, 6, Merck/EMD Serono, 1, Momenta Pharmaceuticals, 2, Nexstone Immunology, 2, Nimbus Lakshmi, 2, Proviant, 2, Sanofi, 2, Sinomab Biosciences, 2, Thermofisher, 5, UCB, 2; E. Ginzler: None; M. Dooley: None; R. Ramsey-Goldman: Ampel Solutions, 2, Calabetta, 2, Exagen, 2, Immunocor, 6; S. Manzi: AbbVie, 5, Allegheny Singer Research Institute, 10, AstraZeneca, 2, 5, Exagen Diagnostics, Inc, 2, 9, 10, GSK, 2, 5, Lilly, 2, Lupus Foundation of America, 4, Novartis, 2, UCB Advisory Board, 2, Univiersity of Pittsburgh, 10; A. Jonsen: None; G. Alarcón: None; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 5, 6, Biogen, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 5, 6, GlaxoSmithKline, 6, Janssen, 2, 6, MSD/Merck Sharp and Dohme, 5, Novartis, 5, Pfizer, 2, 5, 6, RemeGen, 2, Roche, 5, Sanofi, 5, UCB, 2, 5, 6; C. Aranow: AstraZeneca, 2, Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, 5, kezar Inc, 2; M. MacKay: None; G. Ruiz-Irastorza: None; S. Lim: None; M. Inanc: None; K. Kalunian: AbbVie/Abbott, 2, Amgen, 5, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb(BMS), 2, Eli Lilly, 2, EquilliumBio, 2, Genentech, 2, Gilead, 2, Janssen, 2, KezarBio, 1, Merck/MSD, 2, Novartis, 2, Pfizer, 2, Remegene, 2, Roche, 2, UCB, 5; S. Jacobsen: None; C. Peschken: AstraZeneca, 2, 5, GSK, 2, 5, Roche, 1, 2; D. Kamen: None; A. Askanase: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Aurinia, 2, Bristol-Myers Squibb, 2, Celgene, 2, Eli Lilly, 2, Genentech, 2, GSK, 2, Idorsia, 2, Janssen, 2, Mallinckrodt, 2, Pfizer, 2, UCB Pharma, 2; J. Buyon: Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, Related Sciences, 1; A. Clarke: AstraZeneca, 2, Bristol-Myers Squibb(BMS), 2, GlaxoSmithKlein(GSK), 2, 5, Otsuka, 2, Roche, 2.



