Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III: SpA
2237: Upadacitinib in Refractory Psoriatic Arthritis. Multicenter Study of 134 Patients in Clinical Practice
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- IU
Abstract Poster Presenter(s)
Eva Galindez-Agirregoikoa1, Diana Prieto-Peña2, Maria Luz Garcia Vivar1, Lucia Vega-Alvarez1, Cristina Vergara3, Irati Urionaguena4, Consuelo Ramos-Giráldez5, Raquel Almodovar6, Beatriz Joven Ibáñez7, Rosario Garcia-Vicuna8, Vega Jovani9, Teresa González10, Àngels Martínez-Ferrer11, Ana Urruticoechea Arana12, Bryan Josue Flores Robles13, Cristina Campos Fernández14, Lilian Maria Lopez Nunez15, Joaquin Maria Belzunegui Otano16, Marina Pavia Pascual17, Esteban Rubio18, Angel Ramos-Calvo19, Noemi Busquets20, Ana Pérez Gómez21, Francisco Miguel Ortiz Sanjuan22, Rafael Benito Melero-Gonzalez23, Cristina Macía24, María Ángeles Puche Larrubia25, Jose Antonio Pinto Tasende26, Cristina Fernandez27, Maria Paz Martinez-Vidal28, Jaime Calvo- Alén29, Emma Beltran-Catalan30, Mireia Moreno31, Silvia Pérez-Barrio32, iñigo Gorostiza Hormaeche1 and Ricardo Blanco33, 1Basurto University Hospital, Bilbao, Spain, 2Hospital Universitario Marqués de Valdecilla, Santander, Spain, 3Hospital Universitario Infanta Sofía, Madrid, Spain, 4GALDAKAO-USANSOLO UNIVERSITY HOSPITAL, GERNIKA-LUMO, Spain, 5Rheumatology Department Hospital Universitario Virgen de Valme, Sevilla, Spain, 6Alcorcón Foundation University Hospital, Madrid, Spain, 7Hospital Universitario 12 de Octubre, Madrid, Spain, 8Hospital Universitario de la Princesa, Madrid, Spain, 9Department of Rheumatology, Hospital General Universitario Dr. Balmis, Alicante, Spain, 10Hospital General Universitario Gregorio Marañón, Madrid, Spain, 11Hospital Universitario Dr Peset Valéncia, Valéncia, Spain, 12Hospital Can Misses, Ibiza, Spain, 13Hospital Universitario San Pedro, Logroño, Spain, 14Hospital General Universitario Valencia, Valencia, Spain, 15Son llatzer, Palmanyola, Spain, 16University Hospital Donostia, Donostia-San Sebasti, Spain, 17Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain, 18Servicio Andaluz Salud, Sevilla, Spain, 19Complejo Hospitalario de Soria, Soria, Spain, 20HOSP. GENERAL DE GRANOLLERS, GRANOLLERS, Spain, 21Rheumatology, Hospital Principe de Asturias, Alcalá de Henares, Spain, 22Hospital Universitario y Politecnico La Fe, Valencia, Spain, 23CHU Vigo, O Carballino, Spain, 24Hospital Universitario Ramón y Cajal, Madrid, Spain, 25Department of Rheumatology, Reina Sofia University Hospital, Cordoba, Spain, 26Rheumatology department, Complexo Hospitalario Universitario A Coruña (CHUAC). Instituto de Investigación Biomédica A Coruña (INIBIC), A Coruña, Spain, 27Hospital Universitario San Juan de Alicante, Alicante, Spain, 28Hospital Universitario San Juan Alicante, Alicante, Spain, 29Rheumatology, Bioaraba Research Unit, Hospital Universitario Araba, Vitoria, Spain, 30HOSPITAL DEL MAR, Barcelona, Spain, 31Parc Tauli Hospital Universitari, I3PT(UAB), Barcelona, Spain, 32Hospital Universitario de Basurto, Bilbao, Spain, 33Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain
Background/Purpose: The EMA authorized Upadacitinib (UPA) in PsA in January 2021. UPA has shown efficacy in PsA refractory to anti-TNF in a clinical trial (RCT). Our objectives are: a) to study the effectiveness and safety of UPA in the 1st clinical practice (RWE) cases in Spain and b) to compare RWE patients with those of RCT.
Methods: Multicenter study of 134 patients with PsA treated with UPA in Spain. The diagnosis of PsA was made using CASPAR criteria. Patients with refractory PsA from 29 Rheumatology Services (January 2021-January 2023) who had received ≥1 dose of UPA (15 mg/d) with at least 1 follow-up visit were included. Refractory PsA was defined if low clinical activity or remission had not been achieved with biological (b) and/or targeted synthetic (ts) DMARDs.
The outcomes were the effectiveness, safety and saving of corticosteroids (CS). A comparative study was carried out between this RWE cohort and those of the SELECT-PsA 2 RCT.
Results are expressed as percentages, mean±SD or median [IQR] depending on the distribution of the variable.
Results: 134 patients (97 women) were studied, mean age 51.8±11.2 years (Table 1). The joint pattern was: peripheral (61.9%), mixed (30.6%) and axial (7.5%). During the evolution, they had also presented enthesitis (35.3%), dactylitis (25.4%), skin involvement (73.9%) and onychopathy (24.4%).
Prior to the UPA, they had received oral CS (68.7%) (mean maximum dose of prednisone 13.4±9.3) and a mean per patient of csDMARDs (1.8±1.0) and b-DMARD (3.3±2.2). The b-DMARDs were: Adalimumab (n=101), Secukinumab (66), Etanercept (53), Ixekizumab (44), Ustekinumab (44), Certolizumab (37), Infliximab (30), Golimumab (26), Guselkumab (2), Abatacept (2), Brodalumab (1). In addition, they received the following ts-DMARDs: Tofacitinib (n=29), Apremilast (27), Filgotinib (1).
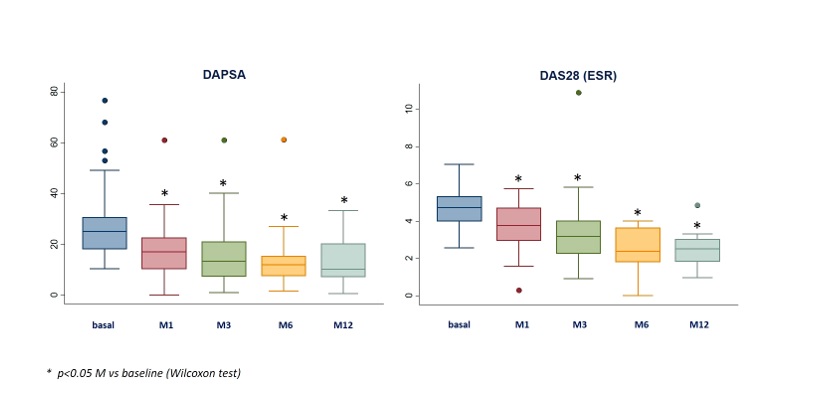
UPA at baseline was associated with: a) prednisone (43.3%; mean dose 8.3±5.6 mg/d). b) csDMARDs (n=64; 47.8%): MTX (n=39), LEF (19), SSZ (10). At the start of the UPA they presented peripheral arthritis (78.4%), axial activity (29.1%), skin involvement (25.4%), onychopathy (11.3%), enthesitis (21.6%) and dactylitis (10.5%). After a mean follow-up of 5.9±5.1 months, a rapid and sustained improvement was observed in the activity indices (table 2, figure) and in the laboratory tests (table 2).
At the 6th month, an improvement in axial involvement (35.7%) and extra-articular manifestations was observed: dactylitis (80%), enthesitis (53.8%) and skin involvement (69.2%), as well as a CS-sparing effect (p=0.031) (table 2).
The RWE patients compared to the RCT were mostly women, refractory to a greater number of previous b-DMARDs and received more concomitant CS (Table 1).
No serious adverse effects (AE) were observed. Minor AE were reported in 23 (17.2%) patients. UPA was discontinued in 44 (32.8%) (28 ineffectiveness, 4 patient decision, 4 infection, 2 de novo anterior uveitis episodes, 1 thrombosis, 1 surgery, 1 pregnancy, 1 urticaria and 1 diarrhea).
Conclusion: In this study, the first patients treated with UPA in PsA in RWE in Spain received more CS simultaneously and were refractory to a greater number of b-DMARDs than those in the RCT. As in the RCT, UPA was effective, fast, and relatively safe in refractory APs in RWE.
.jpg)
.jpg)
E. Galindez-Agirregoikoa: None; D. Prieto-Peña: None; M. Garcia Vivar: None; L. Vega-Alvarez: None; C. Vergara: None; I. Urionaguena: None; C. Ramos-Giráldez: None; R. Almodovar: None; B. Joven Ibáñez: None; R. Garcia-Vicuna: None; V. Jovani: None; T. González: None; À. Martínez-Ferrer: None; A. Urruticoechea Arana: None; B. Flores Robles: None; C. Campos Fernández: None; L. Lopez Nunez: None; J. Belzunegui Otano: None; M. Pavia Pascual: None; E. Rubio: None; A. Ramos-Calvo: None; N. Busquets: None; A. Pérez Gómez: None; F. Ortiz Sanjuan: None; R. Melero-Gonzalez: None; C. Macía: None; M. Puche Larrubia: None; J. Pinto Tasende: None; C. Fernandez: None; M. Martinez-Vidal: None; J. Calvo- Alén: AbbVie, 2, AstraZeneca, 2, Biogen, 6, BMS, 5, Galapagos, 6, GSK, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Roche, 5, Sanofi, 2; E. Beltran-Catalan: None; M. Moreno: None; S. Pérez-Barrio: None; i. Gorostiza Hormaeche: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.
Background/Purpose: The EMA authorized Upadacitinib (UPA) in PsA in January 2021. UPA has shown efficacy in PsA refractory to anti-TNF in a clinical trial (RCT). Our objectives are: a) to study the effectiveness and safety of UPA in the 1st clinical practice (RWE) cases in Spain and b) to compare RWE patients with those of RCT.
Methods: Multicenter study of 134 patients with PsA treated with UPA in Spain. The diagnosis of PsA was made using CASPAR criteria. Patients with refractory PsA from 29 Rheumatology Services (January 2021-January 2023) who had received ≥1 dose of UPA (15 mg/d) with at least 1 follow-up visit were included. Refractory PsA was defined if low clinical activity or remission had not been achieved with biological (b) and/or targeted synthetic (ts) DMARDs.
The outcomes were the effectiveness, safety and saving of corticosteroids (CS). A comparative study was carried out between this RWE cohort and those of the SELECT-PsA 2 RCT.
Results are expressed as percentages, mean±SD or median [IQR] depending on the distribution of the variable.
Results: 134 patients (97 women) were studied, mean age 51.8±11.2 years (Table 1). The joint pattern was: peripheral (61.9%), mixed (30.6%) and axial (7.5%). During the evolution, they had also presented enthesitis (35.3%), dactylitis (25.4%), skin involvement (73.9%) and onychopathy (24.4%).
Prior to the UPA, they had received oral CS (68.7%) (mean maximum dose of prednisone 13.4±9.3) and a mean per patient of csDMARDs (1.8±1.0) and b-DMARD (3.3±2.2). The b-DMARDs were: Adalimumab (n=101), Secukinumab (66), Etanercept (53), Ixekizumab (44), Ustekinumab (44), Certolizumab (37), Infliximab (30), Golimumab (26), Guselkumab (2), Abatacept (2), Brodalumab (1). In addition, they received the following ts-DMARDs: Tofacitinib (n=29), Apremilast (27), Filgotinib (1).
UPA at baseline was associated with: a) prednisone (43.3%; mean dose 8.3±5.6 mg/d). b) csDMARDs (n=64; 47.8%): MTX (n=39), LEF (19), SSZ (10). At the start of the UPA they presented peripheral arthritis (78.4%), axial activity (29.1%), skin involvement (25.4%), onychopathy (11.3%), enthesitis (21.6%) and dactylitis (10.5%). After a mean follow-up of 5.9±5.1 months, a rapid and sustained improvement was observed in the activity indices (table 2, figure) and in the laboratory tests (table 2).
At the 6th month, an improvement in axial involvement (35.7%) and extra-articular manifestations was observed: dactylitis (80%), enthesitis (53.8%) and skin involvement (69.2%), as well as a CS-sparing effect (p=0.031) (table 2).
The RWE patients compared to the RCT were mostly women, refractory to a greater number of previous b-DMARDs and received more concomitant CS (Table 1).
No serious adverse effects (AE) were observed. Minor AE were reported in 23 (17.2%) patients. UPA was discontinued in 44 (32.8%) (28 ineffectiveness, 4 patient decision, 4 infection, 2 de novo anterior uveitis episodes, 1 thrombosis, 1 surgery, 1 pregnancy, 1 urticaria and 1 diarrhea).
Conclusion: In this study, the first patients treated with UPA in PsA in RWE in Spain received more CS simultaneously and were refractory to a greater number of b-DMARDs than those in the RCT. As in the RCT, UPA was effective, fast, and relatively safe in refractory APs in RWE.
.jpg)
TABLE 1. Baseline characteristics
.jpg)
TABLE 2. Evolution

Figure
E. Galindez-Agirregoikoa: None; D. Prieto-Peña: None; M. Garcia Vivar: None; L. Vega-Alvarez: None; C. Vergara: None; I. Urionaguena: None; C. Ramos-Giráldez: None; R. Almodovar: None; B. Joven Ibáñez: None; R. Garcia-Vicuna: None; V. Jovani: None; T. González: None; À. Martínez-Ferrer: None; A. Urruticoechea Arana: None; B. Flores Robles: None; C. Campos Fernández: None; L. Lopez Nunez: None; J. Belzunegui Otano: None; M. Pavia Pascual: None; E. Rubio: None; A. Ramos-Calvo: None; N. Busquets: None; A. Pérez Gómez: None; F. Ortiz Sanjuan: None; R. Melero-Gonzalez: None; C. Macía: None; M. Puche Larrubia: None; J. Pinto Tasende: None; C. Fernandez: None; M. Martinez-Vidal: None; J. Calvo- Alén: AbbVie, 2, AstraZeneca, 2, Biogen, 6, BMS, 5, Galapagos, 6, GSK, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Roche, 5, Sanofi, 2; E. Beltran-Catalan: None; M. Moreno: None; S. Pérez-Barrio: None; i. Gorostiza Hormaeche: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6.



