Poster Session B
Rheumatoid arthritis (RA)
Session: (1308–1344) RA – Treatment Poster II
1308: Certolizumab Pegol Shows Longer Retention Rate in Comparison with Other TNF Inhibitors in Patients with Rheumatoid Arthritis and High Rheumatoid Factor Titers at Baseline. a Multicentre and Retrospective Study
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.jpg)
Clementina Lopez-Medina, MD
Reina Sofia University Hospital
Cordoba, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Clementina López Medina1, Jerusalem Calvo2, Maria del Carmen Abalos-Aguilera3, Francisco Cepas4, Chamaida Plasencia-Rodríguez5, Ana Martinez Feito6, Alejandro Balsa5, Regina Faré-García7, Antonio Juan Mas7, Virginia Ruiz-Esquide8, Luis Sainz-Comas9, César Díaz-Torné9, Javier Godoy10, Isabel Anon Onate11, Natalia Mena Vazquez12, SARA MANRIQUE13, Marina soledad Moreno Garcia14, Rafaela Ortega Castro15 and Alejandro Escudero-Contreras16, 1Rheumatology Department, Cochin Hospital; INSERM (U1153): Clinical Epidemiology and Biostatistics, University of Paris; Rheumatology Department, Reina Sofia Hospital, Cordoba / IMIBIC / University of Cordoba, Cordoba, Spain, 2Reina Sofia University Hospital, Córdoba, Spain, 3Rheumatology Department, Reina Sofia University Hospital/Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Córdoba, Spain, 4Maimonides Institute for Biomedical Research (IMIBIC), Córdoba, Spain, 5Hospital Universitario La Paz, Madrid, Spain, 6Immuno-Rheumatology research group , Institute for Health Research (IdiPAZ), Madrid, Spain, 7Son Llazter University Hospital, Palma, Spain, 8Hospital Clinic, Rheumatology, Barcelona, Spain, 9Hospital de Santa Creu i Sant Pau, Barcelona, Spain, 10Jaen University Hospital, Jaen, Spain, 11Hospital Universitario de Jaen, Jaen, Spain, 12IBIMA, Málaga, Spain, 13Division of Rheumatology, Hospital Regional Universitario Carlos Haya, Malaga, Spain, 14Hospital Miguel Servet, Zaragoza, Spain, 15Hospital Reina Sofía, Cordoba, Spain, 16Rheumatology Department, Reina Sofia University Hospital, Cordoba/Maimonides Biomedical Research Institute of Cordoba (IMIBIC)/University of Cordoba, Córdoba, Spain
Background/Purpose: Rheumatoid Factor (RF) is an antibody against the Fc fragment of IgG that contributes to the Rheumatoid Arthritis (RA) development. RF can bind the Fc fragment of infliximab, suggesting the hypothesis that it can also bind other monoclonal antibodies (mAB (Adalimumab, Golimumab and Infliximab)) and fusion proteins (FP (Etanercept)), leading to a lower drug levels and potential early withdrawal. Conversely, the absence of Fc fragment in Certolizumab Pegol (PEG) may lead to a higher retention rate in comparison with other drugs in RA patients (pts) with high RF titers. Objectives: a) to compare the retention rate to PEG vs. mAB and vs. FP in AR pts with high RF titers; b) to conduct the similar analysis but stratifying in naïve and non-naïve pts.
Methods: Longitudinal, retrospective and multicentre study including pts with RA and treated with any TNFi (mAB, FP or PEG) between 2010-2022. RF levels before TNFi initiation and the dates of both initiation and treatment withdrawal were collected. Log-rank test and Kaplan-Meier curves were conducted to evaluate the retention rate to the three molecular structures according to the level of RF considering the quartiles of the baseline titres as cut-offs: < 15, >60 and >200 UI/ml (negative, high, and very high levels, respectively). A sensitivity analysis was performed using a Propensity Score (PS) matching the PEG, mAB and FP populations according to the age, sex and previous TNFi use.
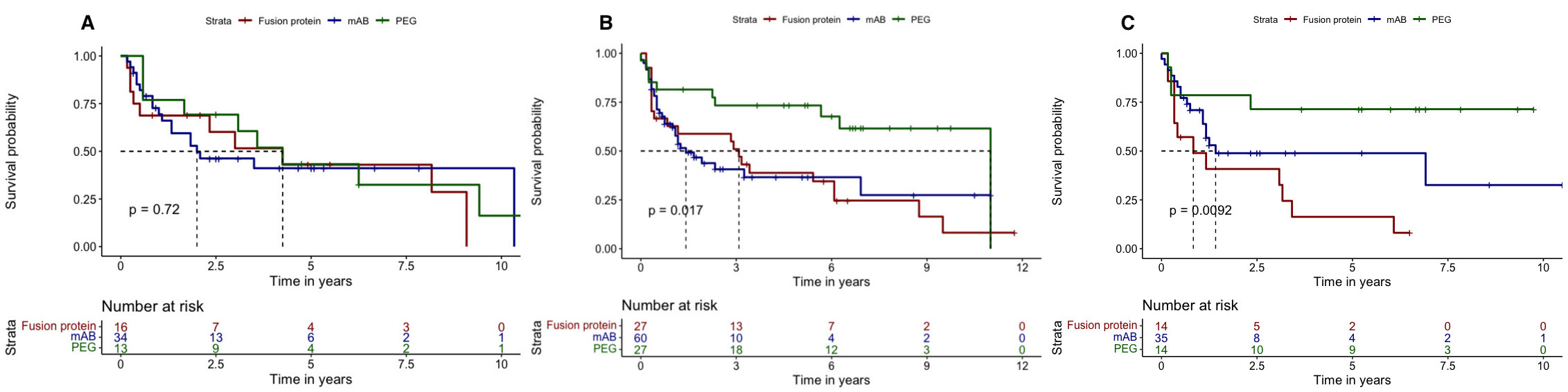
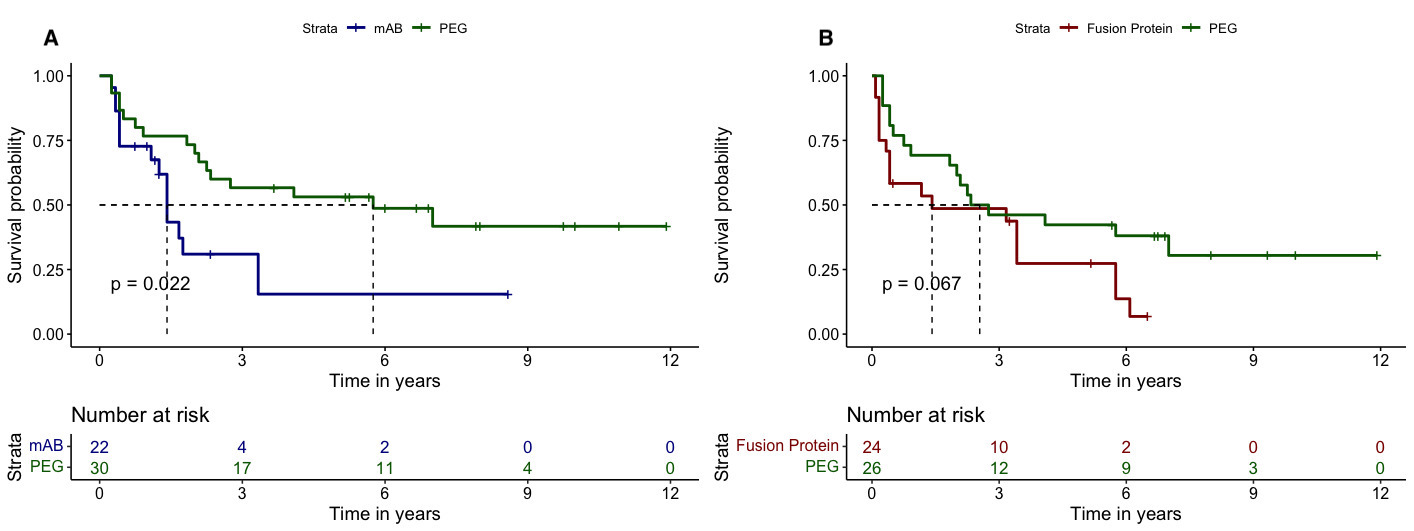
Results: A total of 755 pts with RA treated with TNFi (132 PEG, 441 mAB and 182 FP) and with available titres of RF were included (mean age 53 (12) years, 80.2% female and 70.7% naïve). In the total population, a similar retention rate to PEG, mAB and FP was found (6.1y (95%CI 3.7-NA), 4.2y (95%CI 3.3-8.7) and 4.6y (95%CI 2.9-7.8), respectively, p=0.380). According to the RF levels, the retention rate to the three molecular structures were similar in seronegative pts(F.1A), and in those with high (F.1B) and very high levels (F.1C). When selecting non-naïve pts, those with negative RF titres showed similar retention rates across molecular structures (F.2A). However, in non-naïve ptswith high levels of RF, those treated with PEG showed a significant longer retention rate in comparison with mAB and FP (median 11.0y (95%CI 6.3-NA), 1.4y (95%CI 1.1-NA) and 3.1y (95%CI 0.8-8.8) respectively, p=0.017)(F.2B). Similar results were obtained in non-naïve ptswith very high titres of RF (F.2C). In ptswith very high levels of RF, the sensitivity analysis using the PS showed a significant longer retention rate to PEG vs. mAB (median 5.8y (95%CI 2.3-NA) vs. 1.4y (95%CI 1.3-NA), respectively, p=0.022) irrespectively of the previous use of TNFi (F.3A). Likewise, PEG vs. FP showed a longer retention rate, although these differences were non-significant (median 2.5y (95%CI 1.8-NA) vs. 1.4y (95%CI 0.4-5.8), p=0.067)(F.3B).
Conclusion: High or very high RF titers before TNFi initiation were associated with a longer retention rate in non-naïve pts treated with PEG in comparison with mAB and FP. When matching the population using PS, very high levels of RF were associated with longer retention rate to PEG vs. mAB irrespectively of the previous use of TNFi. These results confirm the possible effect of the RF in binding the Fc fragment of the drug.
.jpg)
C. López Medina: AbbVie, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 6, MSD, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; J. Calvo: None; M. Abalos-Aguilera: None; F. Cepas: None; C. Plasencia-Rodríguez: Abbvie, 5, 6, Eli Lilly, 6, Novartis, 5, Pfizer, 5, 6, Roche, 6; A. Martinez Feito: None; A. Balsa: AbbVie/Abbott, 1, 2, 5, 6, Bristol-Myers Squibb(BMS), 1, 5, Eli Lilly, 1, 5, 6, Merck/MSD, 1, 5, Novartis, 5, Pfizer, 1, 5, 6, UCB, 1, 5, 6; R. Faré-García: None; A. Juan Mas: None; V. Ruiz-Esquide: None; L. Sainz-Comas: None; C. Díaz-Torné: None; J. Godoy: None; I. Anon Onate: None; N. Mena Vazquez: None; S. MANRIQUE: None; M. Moreno Garcia: None; R. Ortega Castro: None; A. Escudero-Contreras: None.
Background/Purpose: Rheumatoid Factor (RF) is an antibody against the Fc fragment of IgG that contributes to the Rheumatoid Arthritis (RA) development. RF can bind the Fc fragment of infliximab, suggesting the hypothesis that it can also bind other monoclonal antibodies (mAB (Adalimumab, Golimumab and Infliximab)) and fusion proteins (FP (Etanercept)), leading to a lower drug levels and potential early withdrawal. Conversely, the absence of Fc fragment in Certolizumab Pegol (PEG) may lead to a higher retention rate in comparison with other drugs in RA patients (pts) with high RF titers. Objectives: a) to compare the retention rate to PEG vs. mAB and vs. FP in AR pts with high RF titers; b) to conduct the similar analysis but stratifying in naïve and non-naïve pts.
Methods: Longitudinal, retrospective and multicentre study including pts with RA and treated with any TNFi (mAB, FP or PEG) between 2010-2022. RF levels before TNFi initiation and the dates of both initiation and treatment withdrawal were collected. Log-rank test and Kaplan-Meier curves were conducted to evaluate the retention rate to the three molecular structures according to the level of RF considering the quartiles of the baseline titres as cut-offs: < 15, >60 and >200 UI/ml (negative, high, and very high levels, respectively). A sensitivity analysis was performed using a Propensity Score (PS) matching the PEG, mAB and FP populations according to the age, sex and previous TNFi use.
Results: A total of 755 pts with RA treated with TNFi (132 PEG, 441 mAB and 182 FP) and with available titres of RF were included (mean age 53 (12) years, 80.2% female and 70.7% naïve). In the total population, a similar retention rate to PEG, mAB and FP was found (6.1y (95%CI 3.7-NA), 4.2y (95%CI 3.3-8.7) and 4.6y (95%CI 2.9-7.8), respectively, p=0.380). According to the RF levels, the retention rate to the three molecular structures were similar in seronegative pts(F.1A), and in those with high (F.1B) and very high levels (F.1C). When selecting non-naïve pts, those with negative RF titres showed similar retention rates across molecular structures (F.2A). However, in non-naïve ptswith high levels of RF, those treated with PEG showed a significant longer retention rate in comparison with mAB and FP (median 11.0y (95%CI 6.3-NA), 1.4y (95%CI 1.1-NA) and 3.1y (95%CI 0.8-8.8) respectively, p=0.017)(F.2B). Similar results were obtained in non-naïve ptswith very high titres of RF (F.2C). In ptswith very high levels of RF, the sensitivity analysis using the PS showed a significant longer retention rate to PEG vs. mAB (median 5.8y (95%CI 2.3-NA) vs. 1.4y (95%CI 1.3-NA), respectively, p=0.022) irrespectively of the previous use of TNFi (F.3A). Likewise, PEG vs. FP showed a longer retention rate, although these differences were non-significant (median 2.5y (95%CI 1.8-NA) vs. 1.4y (95%CI 0.4-5.8), p=0.067)(F.3B).
Conclusion: High or very high RF titers before TNFi initiation were associated with a longer retention rate in non-naïve pts treated with PEG in comparison with mAB and FP. When matching the population using PS, very high levels of RF were associated with longer retention rate to PEG vs. mAB irrespectively of the previous use of TNFi. These results confirm the possible effect of the RF in binding the Fc fragment of the drug.
.jpg)
Figure 1. Retention rate to the three molecular structures according to the baseline RF titers: (A) <15 IU/ml, (B) >60 IU/ml and (C) >200 UI/ml.

Figure 2. Retention rate to the three molecular structures according to the baseline RF titers in non-naïve patients: (A) <15 IU/ml, (B) >60 IU/ml and (C) >200 UI/ml.

Figure 3. Sensitivity analysis in patients with very high baseline levels of RF (>200 UI/ml) using the PS: (A) PEG vs. mAB and (B) PEG vs. fusion protein.
C. López Medina: AbbVie, 5, 6, Eli Lilly, 2, 5, 6, Janssen, 6, MSD, 6, Novartis, 2, 5, 6, UCB Pharma, 2, 5, 6; J. Calvo: None; M. Abalos-Aguilera: None; F. Cepas: None; C. Plasencia-Rodríguez: Abbvie, 5, 6, Eli Lilly, 6, Novartis, 5, Pfizer, 5, 6, Roche, 6; A. Martinez Feito: None; A. Balsa: AbbVie/Abbott, 1, 2, 5, 6, Bristol-Myers Squibb(BMS), 1, 5, Eli Lilly, 1, 5, 6, Merck/MSD, 1, 5, Novartis, 5, Pfizer, 1, 5, 6, UCB, 1, 5, 6; R. Faré-García: None; A. Juan Mas: None; V. Ruiz-Esquide: None; L. Sainz-Comas: None; C. Díaz-Torné: None; J. Godoy: None; I. Anon Onate: None; N. Mena Vazquez: None; S. MANRIQUE: None; M. Moreno Garcia: None; R. Ortega Castro: None; A. Escudero-Contreras: None.



