Poster Session C
Metabolic bone disease
Session: (1996–2018) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
2000: Efficacy of Romosozumab for Glucocorticoid-induced Osteoporosis in Patients with Rheumatic Diseases; A Prospective Study
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MK
Mai Kawazoe, MD, PhD
Division of Rheumatology, Department of Internal Medicine, Toho University School of Medicine
Tokyo, JapanDisclosure information not submitted.
Abstract Poster Presenter(s)
Mai Kawazoe1, Kaichi Kaneko2, Shotaro Masuoka3, Soichi yamada3, Zento Yamada3, Sei Muraoka3, Karin Furukawa1, Hiroshi Sato3, Eri Watanabe3, Keiko Koshiba3, Izumi Irita3, Miwa Kanaji3 and Toshihiro Nanki1, 1Division of Rheumatology, Department of Internal Medicine, Toho University School of Medicine, Ota-ku, Japan, 2Division of Rheumatology, Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan, 3Division of Rheumatology, Department of Internal Medicine, Toho University School of Medicine, Tokyo, Japan
Background/Purpose: Glucocorticoids are widely used to treat a variety of diseases, including rheumatic diseases. Although glucocorticoids improve the outcome for these diseases, various side effects of long-term treatment, such as osteoporosis, have become an important problem. Although the effects of glucocorticoids on bone metabolism remain unclear, Wnt signaling is now known to be involved in bone formation and resorption. We previously found that glucocorticoid therapy caused increase serum sclerostin, which inhibits Wnt signaling, and decrease Wnt3a, suggesting that suppression of Wnt/β-catenin signaling pathway might contribute glucocorticoid-induced osteoporosis (GIO). Therefore, inhibition of sclerostin could be a treatment for GIO. Romosozumab (ROMO) is a monoclonal antibody against sclerostin. Efficacy of ROMO in patients with postmenopausal osteoporosis and men with osteoporosis has been demonstrated in clinical trials. However, the effectiveness of ROMO in GIO is not clear. The purpose of this study was to evaluate the efficacy of ROMO compared to existing therapy by measuring bone mineral density (BMD) in patients recently initiated on glucocorticoid therapy.
Methods: This is a randomized, prospective, interventional study. Patients with rheumatic diseases who had not previously received osteoporosis treatment and were newly treated with prednisolone (PSL) 15 mg/day or more were randomly assigned to receive either ROMO, denosumab (DMAb), or bisphosphonate (BP). They were stratified at randomization according to age, sex, dose of PSL and T-scores of the lumbar spine or femoral neck. We measured BMD of the lumbar spine (L2-L4) and femoral neck at 0, 6, 12 months and serum bone turnover markers at 0, 3, 6, 9 and 12 months after initiation of glucocorticoid therapy.
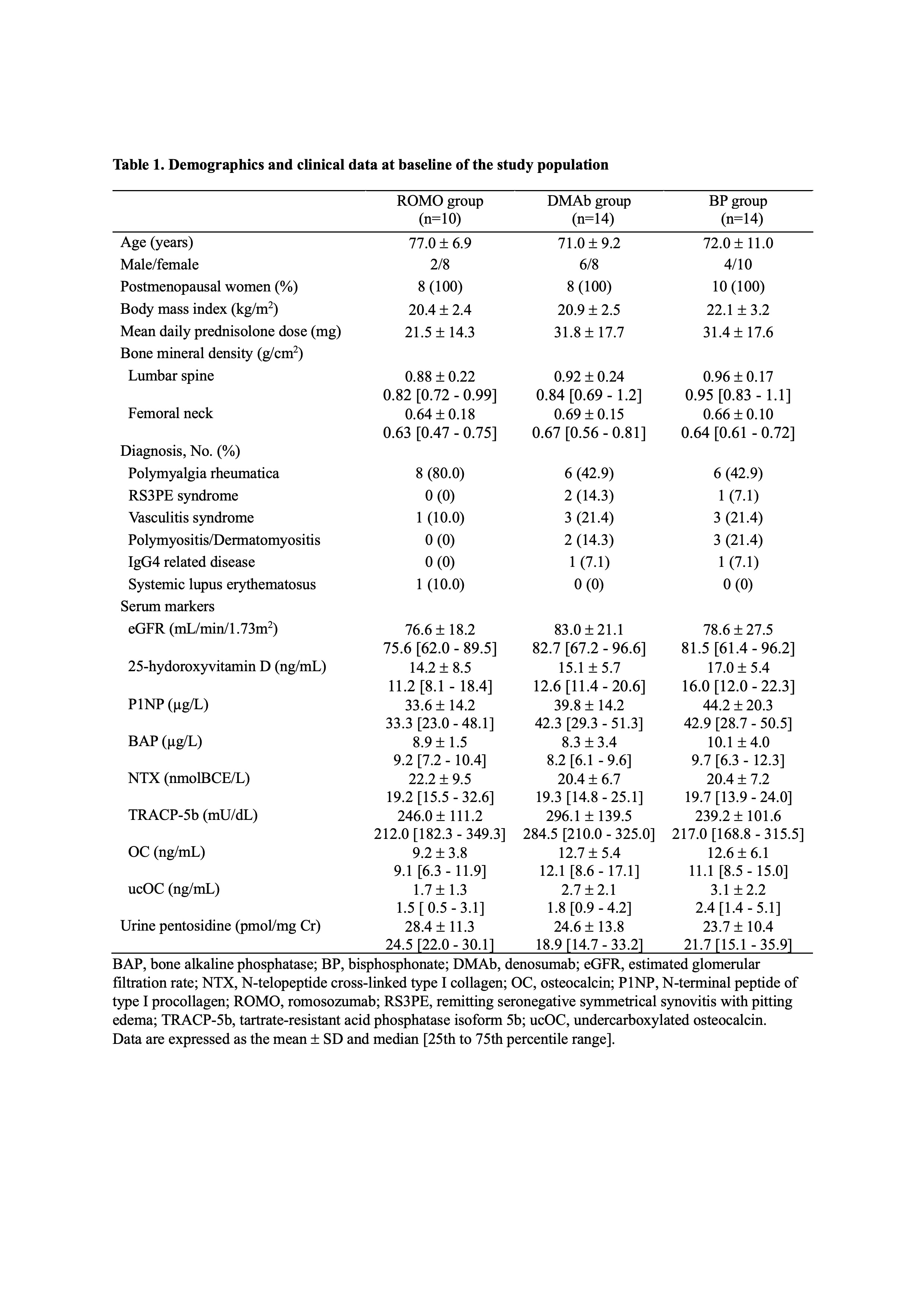
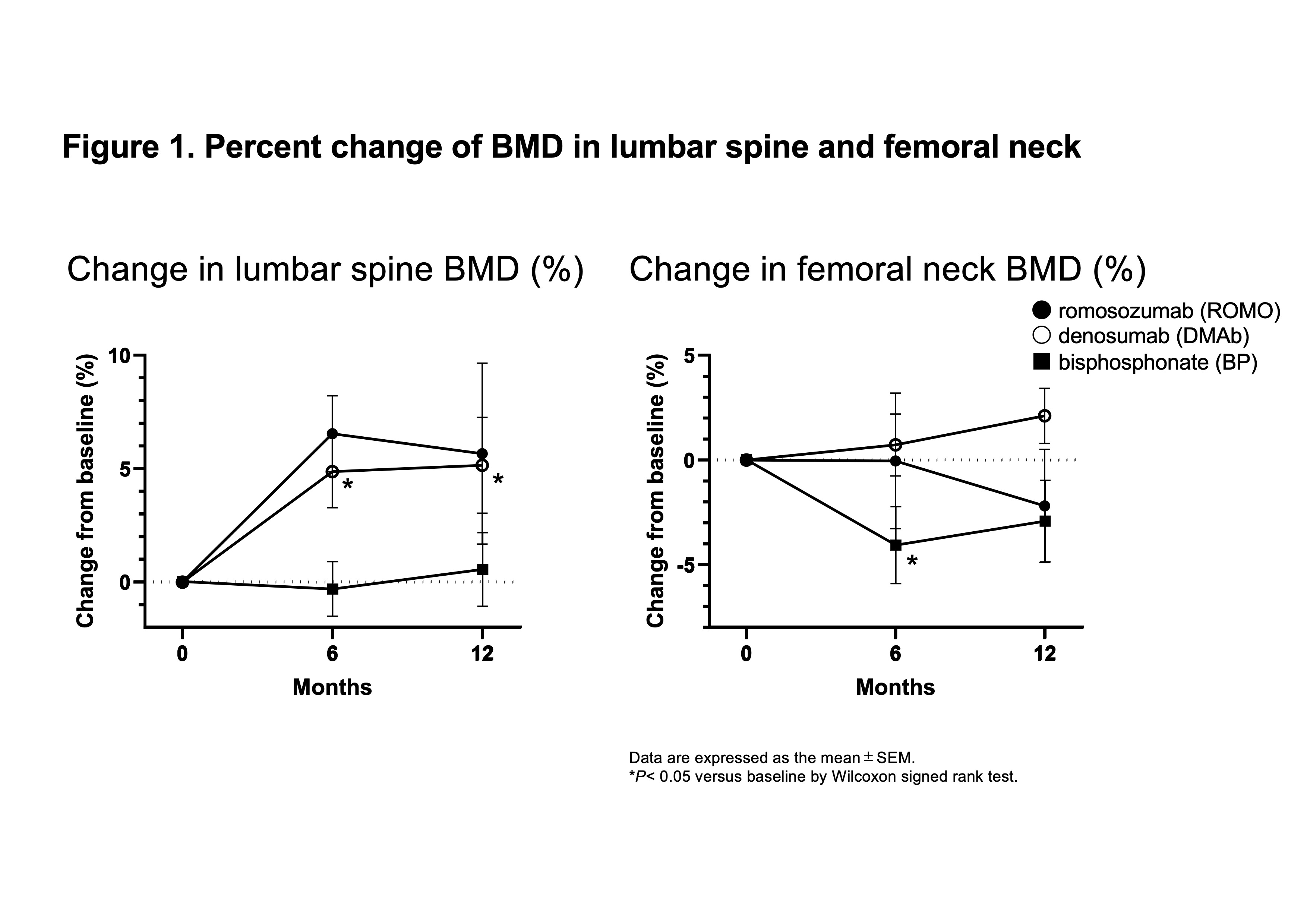
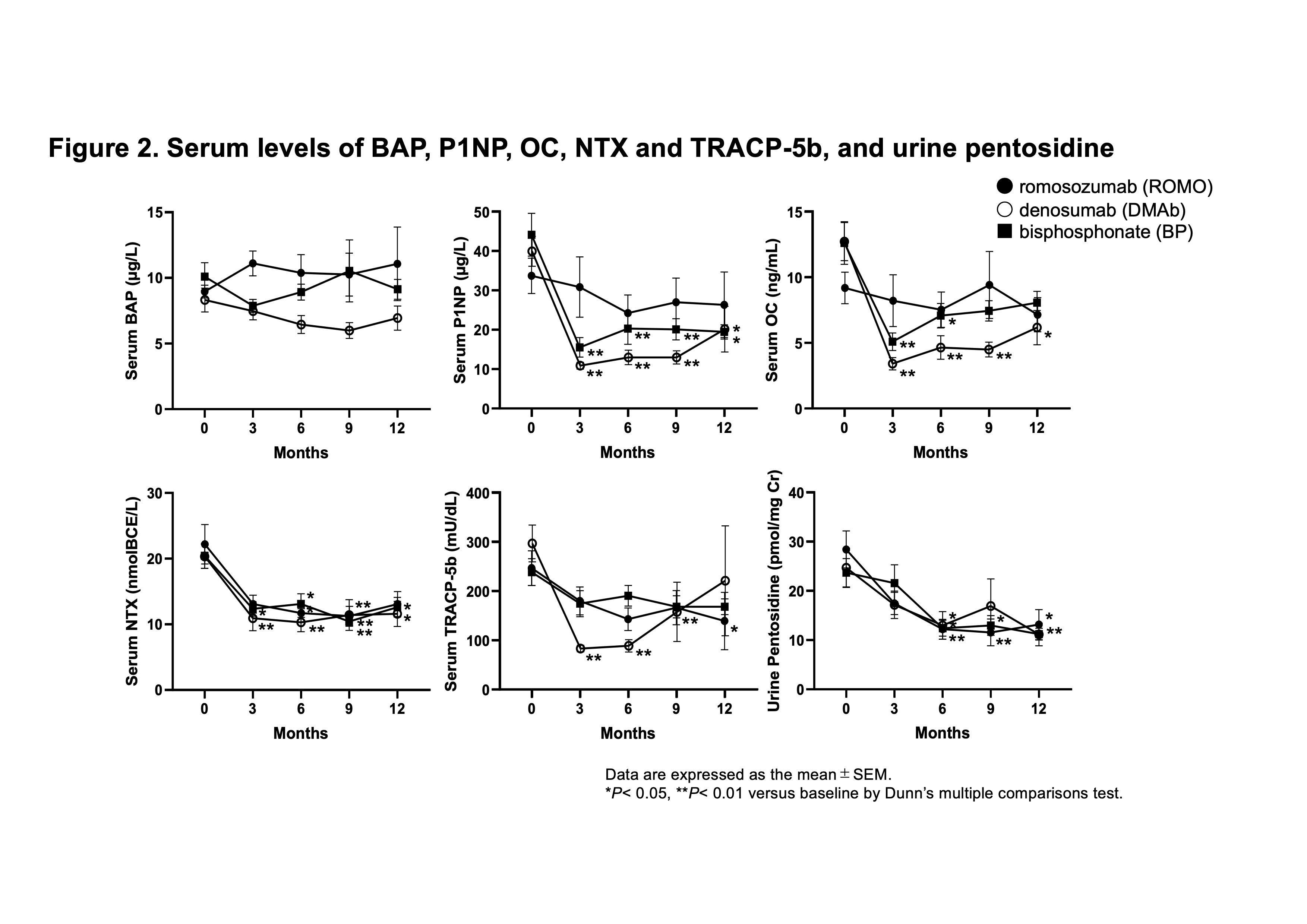
Results: Ten patients were assigned to the ROMO group, 14 to the DMAb group, and 14 to the BP group. Base line data were shown in Table 1. The mean percent change of lumbar spine BMD from baseline at 12 months was greatest for the ROMO group among the three groups (ROMO; 5.7 ± 9.8%, DMAb; 5.2 ± 6.0%, BP; 0.5 ± 5.1%) (Figure 1). The mean change of femoral neck BMD at 12 months was greatest for the DMAb group (ROMO; -2.2 ± 6.0%, DMAb; 2.1 ± 3.7%, BP; -2.9 ± 5.8%). Serum bone alkaline phosphatase (BAP) level, a marker of bone formation, slightly increased in the ROMO group, but decreased in the DMAb group (Figure 2). Serum N-terminal propeptide of type I procollagen (P1NP) and osteocalcin (OC), other bone formation markers, decreased in all three groups, but the change was smaller in the ROMO group. Serum bone resorption markers, N-telopeptide crosslinked of type I collagen (NTX) and tartrate-resistant acid phosphatase isoform 5b (TRACP-5b) decreased in all groups. Urine pentosidine, bone matrix-related marker, decreased in all groups.
Conclusion: Even under treatment with PSL, patients treated with ROMO had increased lumbar spine BMD, but not femoral neck BMD, compared to patients receiving existing osteoporosis treatment with DMAb or BP. This is the first study to analyze the effect of ROMO on GIO, but further validation in a large number of cases is needed.
M. Kawazoe: Asahi Kasei Pharma Corp, 6, AstraZeneca, 6, Ayumi Pharmaceutical Corporation, 6, GlaxoSmithKlein(GSK), 6; K. Kaneko: AbbVie/Abbott, 6, AstraZeneca, 6, Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Gilead, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Novartis, 6, Pfizer, 6, UCB, 6; S. Masuoka: None; S. yamada: None; Z. Yamada: Janssen, 6; S. Muraoka: None; K. Furukawa: None; H. Sato: None; E. Watanabe: GlaxoSmithKlein(GSK), 5; K. Koshiba: None; I. Irita: None; M. Kanaji: None; T. Nanki: AbbVie GK, 5, 6, Asahikasei Pharma Corp., 5, 6, Astellas Pharma Inc., 6, AstraZeneca K.K., 6, Ayumi Pharmaceutical Co., 5, 6, Bristol-Myers Squibb K.K., 5, Chugai Pharmaceutical Co., 2, 5, 6, Eisai Co.,Ltd., 5, 6, Eli Lilly Japan K.K., 5, 6, GlaxoSmithKline plc., 6, Janssen Pharmaceutical K.K., 6, Mitsubishi-Tanabe Pharma Co., 5, 6, Mochida Pharmaceutical Co., Ltd., 5, 6, Nippon Boehringer Ingelheim Co., Ltd., 5, 6, Nippon Kayaku Co., Ltd., 5, Ono Pharmaceutical Co., Ltd., 5, 6, Pfizer Japan Inc., 6, Shionogi & Co., Ltd., 5, Taisho Pharmaceutical Co.,Ltd., 5, Takeda Pharmaceutical Co., Ltd., 6, Teijin Pharma Ltd., 5, UCB Japan Co. Ltd., 1, 6.
Background/Purpose: Glucocorticoids are widely used to treat a variety of diseases, including rheumatic diseases. Although glucocorticoids improve the outcome for these diseases, various side effects of long-term treatment, such as osteoporosis, have become an important problem. Although the effects of glucocorticoids on bone metabolism remain unclear, Wnt signaling is now known to be involved in bone formation and resorption. We previously found that glucocorticoid therapy caused increase serum sclerostin, which inhibits Wnt signaling, and decrease Wnt3a, suggesting that suppression of Wnt/β-catenin signaling pathway might contribute glucocorticoid-induced osteoporosis (GIO). Therefore, inhibition of sclerostin could be a treatment for GIO. Romosozumab (ROMO) is a monoclonal antibody against sclerostin. Efficacy of ROMO in patients with postmenopausal osteoporosis and men with osteoporosis has been demonstrated in clinical trials. However, the effectiveness of ROMO in GIO is not clear. The purpose of this study was to evaluate the efficacy of ROMO compared to existing therapy by measuring bone mineral density (BMD) in patients recently initiated on glucocorticoid therapy.
Methods: This is a randomized, prospective, interventional study. Patients with rheumatic diseases who had not previously received osteoporosis treatment and were newly treated with prednisolone (PSL) 15 mg/day or more were randomly assigned to receive either ROMO, denosumab (DMAb), or bisphosphonate (BP). They were stratified at randomization according to age, sex, dose of PSL and T-scores of the lumbar spine or femoral neck. We measured BMD of the lumbar spine (L2-L4) and femoral neck at 0, 6, 12 months and serum bone turnover markers at 0, 3, 6, 9 and 12 months after initiation of glucocorticoid therapy.
Results: Ten patients were assigned to the ROMO group, 14 to the DMAb group, and 14 to the BP group. Base line data were shown in Table 1. The mean percent change of lumbar spine BMD from baseline at 12 months was greatest for the ROMO group among the three groups (ROMO; 5.7 ± 9.8%, DMAb; 5.2 ± 6.0%, BP; 0.5 ± 5.1%) (Figure 1). The mean change of femoral neck BMD at 12 months was greatest for the DMAb group (ROMO; -2.2 ± 6.0%, DMAb; 2.1 ± 3.7%, BP; -2.9 ± 5.8%). Serum bone alkaline phosphatase (BAP) level, a marker of bone formation, slightly increased in the ROMO group, but decreased in the DMAb group (Figure 2). Serum N-terminal propeptide of type I procollagen (P1NP) and osteocalcin (OC), other bone formation markers, decreased in all three groups, but the change was smaller in the ROMO group. Serum bone resorption markers, N-telopeptide crosslinked of type I collagen (NTX) and tartrate-resistant acid phosphatase isoform 5b (TRACP-5b) decreased in all groups. Urine pentosidine, bone matrix-related marker, decreased in all groups.
Conclusion: Even under treatment with PSL, patients treated with ROMO had increased lumbar spine BMD, but not femoral neck BMD, compared to patients receiving existing osteoporosis treatment with DMAb or BP. This is the first study to analyze the effect of ROMO on GIO, but further validation in a large number of cases is needed.

Demographics and clinical data at baseline of the study population

Percent change of BMD in lumbar spine and femoral neck

Serum levels of BAP, P1NP, OC, NTX and TRACP-5b, and urine pentosidine
M. Kawazoe: Asahi Kasei Pharma Corp, 6, AstraZeneca, 6, Ayumi Pharmaceutical Corporation, 6, GlaxoSmithKlein(GSK), 6; K. Kaneko: AbbVie/Abbott, 6, AstraZeneca, 6, Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Eli Lilly, 6, Gilead, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Novartis, 6, Pfizer, 6, UCB, 6; S. Masuoka: None; S. yamada: None; Z. Yamada: Janssen, 6; S. Muraoka: None; K. Furukawa: None; H. Sato: None; E. Watanabe: GlaxoSmithKlein(GSK), 5; K. Koshiba: None; I. Irita: None; M. Kanaji: None; T. Nanki: AbbVie GK, 5, 6, Asahikasei Pharma Corp., 5, 6, Astellas Pharma Inc., 6, AstraZeneca K.K., 6, Ayumi Pharmaceutical Co., 5, 6, Bristol-Myers Squibb K.K., 5, Chugai Pharmaceutical Co., 2, 5, 6, Eisai Co.,Ltd., 5, 6, Eli Lilly Japan K.K., 5, 6, GlaxoSmithKline plc., 6, Janssen Pharmaceutical K.K., 6, Mitsubishi-Tanabe Pharma Co., 5, 6, Mochida Pharmaceutical Co., Ltd., 5, 6, Nippon Boehringer Ingelheim Co., Ltd., 5, 6, Nippon Kayaku Co., Ltd., 5, Ono Pharmaceutical Co., Ltd., 5, 6, Pfizer Japan Inc., 6, Shionogi & Co., Ltd., 5, Taisho Pharmaceutical Co.,Ltd., 5, Takeda Pharmaceutical Co., Ltd., 6, Teijin Pharma Ltd., 5, UCB Japan Co. Ltd., 1, 6.



