Poster Session C
Systemic lupus erythematosus (SLE)
Session: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
2261: Burden of Flare and Organ Damage in Systemic Lupus Erythematosus (SLE) in the Asia Pacific Region: A Multicenter Cohort Study
Tuesday, November 14, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
.png)
Eric Morand, MD, PhD
Monash University
Clayton, Victoria, AustraliaDisclosure information not submitted.
Abstract Poster Presenter(s)
Rangi Kandane-Rathnayake1, Dominique Milea2, Worawit Louthrenoo3, Alberta Hoi4, Vera Golder1, Jiacai Cho5, Aisha Lateef5, Shue-Fen Luo6, Yeong-Jian J Wu7, Laniyati Hamijoyo8, Sargunan Sockalingam9, Zhanguo Li10, Sandra Navarra11, Leonid Zamora11, Masayoshi Harigai12, Yasuhiro Katsumata12, Madelynn Chan13, Yanjie Hao14, Zhuoli Zhang15, Sean O’Neill16, Fiona Goldblatt17, Shereen Oon18, Xiaomeng Xu2, Aldo Amador Navarro Rojas19, Sang-Cheol Bae20, Chak Sing Lau21, Mandana Nikpour22 and Eric Morand23, 1Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Clayton, Australia, 2GlaxoSmithKline, Value Evidence and Outcomes, Singapore, Singapore, 3Chiang Mai University Hospital, Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai, Thailand, 4Monash University, Department of Medicine, Sub-faculty of Clinical and Molecular Medicine, Melbourne, Australia, 5National University Hospital, Rheumatology Division, Department of Medicine, Singapore, Singapore, 6Chang Gung University, Department of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Taoyuan, Taiwan, 7Chang Gung University, Department of Rheumatology, Allergy and Immunology, Chang Gung Memorial Hospital, Keelung, Taiwan, 8Padjadjaran University/Hasan Sadikin General Hospital, Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Bandung, Indonesia, 9University of Malaya, Department of Medicine, Faculty of Medicine Building, Kuala Lumpur, Malaysia, 10Peking University Health Science Center, Department of Rheumatology and Immunology, People's Hospital, Beijing, China, 11University of Santo Tomas Hospital, Joint and Bone Center, Manila, Philippines, 12Tokyo Women's Medical University, Division of Rheumatology, Department of Internal Medicine, Tokyo, Japan, 13Tan Tock Seng Hospital, Department of Rheumatology, Allergy & Immunology, Singapore, Singapore, 14St Vincent’s Hospital Melbourne, Department of Rheumatology, Melbourne, Australia, 15Peking University First Hospital, Rheumatology and Immunology Department, Beijing, China, 16Department of Medicine, University of New South Wales, Kensington, Australia, 17Royal Adelaide Hospital and Flinders Medical Centre, Adelaide, Australia, 18University of Melbourne at St Vincent’s Hospital, Departments of Rheumatology and Medicine, Fitzroy, Australia, 19GlaxoSmithKline, Medical Affairs, Singapore, Singapore, 20Hanyang University Hospital for Rheumatic Diseases and Hanyang University Institute for Rheumatology Research, Department of Rheumatology, Seoul, South Korea, 21University of Hong Kong, Division of Rheumatology & Clinical Immunology, Department of Medicine, Queen Mary Hospital, Hong Kong, Hong Kong, 22The University of Melbourne at St. Vincent’s Hospital Melbourne, Departments of Medicine and Rheumatology, Melbourne, Australia, 23Monash University, Centre for Inflammatory Diseases, Melbourne, Australia
Background/Purpose: Up to 50% of patients with SLE develop irreversible organ damage within 10 years of diagnosis, and most experience recurrent disease flares of varying severity. Both the prevalence and severity of SLE are known to be higher in patients living in the Asia Pacific region, but few data on flare and damage rates are available. We examined the prevalence of organ damage and flare and estimated the magnitude of longitudinal associations between flares and subsequent organ damage accrual using real-world data from the Asia Pacific region.
Methods: Adult patients with SLE enrolled in a multinational cohort with a minimum of 3 years of observational data, captured prospectively between 2013 and 2020, were studied. Flares were assessed at each clinic visit using the Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI). Flare Index and organ damage was assessed annually using SLICC/ACR Damage Index (SDI). Organ damage was defined as present if the SDI was greater than zero (SDI >0) while damage accrual was defined if the change in SDI was greater than zero (ΔSDI >0). Multivariable, multifailure survival analyses were carried out to quantify the association between flares and organ damage accrual.
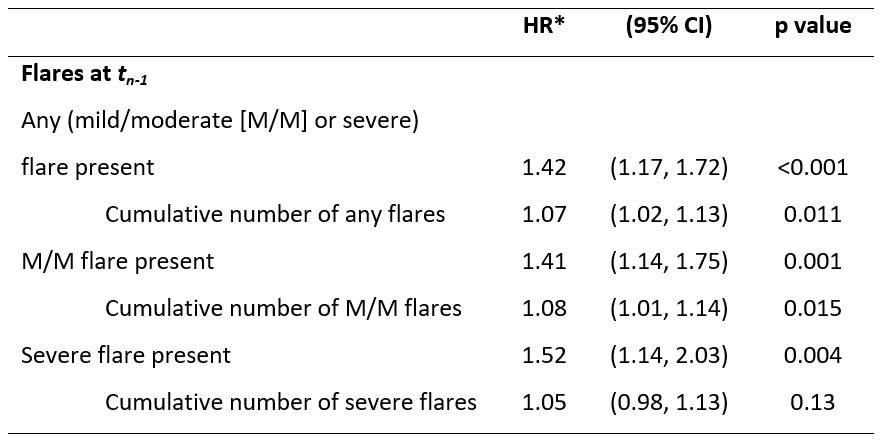
Results: Overall, 1556 patients with SLE, recruited from Australia, China, Indonesia, Japan, Korea, Malaysia, Philippines, Singapore, Taiwan and Thailand, and followed for a median (interquartile range) of 5.7 (3.9, 7.0) years were studied. The prevalence of organ damage at study enrollment (baseline) was 39% (n=614/1556) and the incidence of damage accrual during observation was ~58 per 1000 patient-years (PY) (n=496/8569.86 PY). 40% (n=247/614) of patients with baseline organ damage and 26% (n=249/942) of patients without baseline organ damage accrued damage during the study period. 74% (n=1153) of patients experienced a flare of any severity (mild/moderate or severe) at least once during the study period; 57% (n=885) experienced recurrent (≥2) flares. Flares independently increased the risk of damage accrual; after controlling for confounders, the risk of organ damage accrual at subsequent visits in patients who experienced any flare was 42% greater than in patients without flares (adjusted hazard ratio [HR] [95% confidence interval, CI]: 1.42 [1.17, 1.72]; Table). The risk of damage accrual was greater if patients had severe flares (HR [95% CI]: 1.52 [1.14, 2.03]). For each additional flare, the risk of damage accrual increased by 7% (HR [95% CI]: 1.07 [1.02, 1.13]).
Conclusion: The burden of organ damage and flares in SLE is substantial, and flares quantifiably increase the risk of damage accrual. Prevention of flares should be a major goal of SLE disease management to minimize permanent organ damage.
Funding: GSK (Study 217856)
R. Kandane-Rathnayake: None; D. Milea: GSK, 3, 11; W. Louthrenoo: None; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6; V. Golder: None; J. Cho: None; A. Lateef: None; S. Luo: None; Y. Wu: None; L. Hamijoyo: None; S. Sockalingam: AstraZeneca, 2, 12, Other - for attending meetings and/or travel, Pfizer, 2, 12, Other - for attending meetings and/or travel, ZP Therapeutics, 2; Z. Li: Abbott, 2, 9, Abbvie, 2, 9, BMS, 2, 9, Celgene, 2, 9, Eli Lilly, 2, 9, GSK, 2, 9, Janssen, 2, 9, MSD, 2, 9, Novartis, 2, 9, Pfizer, 2, 9, Roche, 2, 9, UCB Pharma, 2, 9; S. Navarra: Astellas, 6, AstraZeneca, 6, Biogen, 2, Boehringer-Ingelheim, 2, GSK, 6, Novartis, 6, Pfizer, 6; L. Zamora: None; M. Harigai: Astellas Pharma, 6, AstraZeneca, 6, GlaxoSmithKlein(GSK), 6, 12, Post marketing surveillence, Novartis, 5; Y. Katsumata: Asahi Kasei Pharma, 6, Astella, 6, AstraZeneca, 6, Chugai, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Mitsubishi Tanabe Pharma Corporation, 6, Pfizer, 6; M. Chan: None; Y. Hao: None; Z. Zhang: None; S. O’Neill: None; F. Goldblatt: None; S. Oon: None; X. Xu: GSK, 3, 11; A. Amador Navarro Rojas: GSK, 3, 11; S. Bae: None; C. Lau: AstraZeneca, 6, 12, external expert for SLE sterring committee 11.2022; M. Nikpour: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, GSK, 2, 6, Janssen Pharmaceuticals, 2, 5, 6; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5.
Background/Purpose: Up to 50% of patients with SLE develop irreversible organ damage within 10 years of diagnosis, and most experience recurrent disease flares of varying severity. Both the prevalence and severity of SLE are known to be higher in patients living in the Asia Pacific region, but few data on flare and damage rates are available. We examined the prevalence of organ damage and flare and estimated the magnitude of longitudinal associations between flares and subsequent organ damage accrual using real-world data from the Asia Pacific region.
Methods: Adult patients with SLE enrolled in a multinational cohort with a minimum of 3 years of observational data, captured prospectively between 2013 and 2020, were studied. Flares were assessed at each clinic visit using the Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI). Flare Index and organ damage was assessed annually using SLICC/ACR Damage Index (SDI). Organ damage was defined as present if the SDI was greater than zero (SDI >0) while damage accrual was defined if the change in SDI was greater than zero (ΔSDI >0). Multivariable, multifailure survival analyses were carried out to quantify the association between flares and organ damage accrual.
Results: Overall, 1556 patients with SLE, recruited from Australia, China, Indonesia, Japan, Korea, Malaysia, Philippines, Singapore, Taiwan and Thailand, and followed for a median (interquartile range) of 5.7 (3.9, 7.0) years were studied. The prevalence of organ damage at study enrollment (baseline) was 39% (n=614/1556) and the incidence of damage accrual during observation was ~58 per 1000 patient-years (PY) (n=496/8569.86 PY). 40% (n=247/614) of patients with baseline organ damage and 26% (n=249/942) of patients without baseline organ damage accrued damage during the study period. 74% (n=1153) of patients experienced a flare of any severity (mild/moderate or severe) at least once during the study period; 57% (n=885) experienced recurrent (≥2) flares. Flares independently increased the risk of damage accrual; after controlling for confounders, the risk of organ damage accrual at subsequent visits in patients who experienced any flare was 42% greater than in patients without flares (adjusted hazard ratio [HR] [95% confidence interval, CI]: 1.42 [1.17, 1.72]; Table). The risk of damage accrual was greater if patients had severe flares (HR [95% CI]: 1.52 [1.14, 2.03]). For each additional flare, the risk of damage accrual increased by 7% (HR [95% CI]: 1.07 [1.02, 1.13]).
Conclusion: The burden of organ damage and flares in SLE is substantial, and flares quantifiably increase the risk of damage accrual. Prevention of flares should be a major goal of SLE disease management to minimize permanent organ damage.
Funding: GSK (Study 217856)

Table. Longitudinal associations between flares and organ damage accrual (ΔSDI>0) at tn.
*HRs adjusted patients age at tn-1, disease duration at tn-1, gross domestic product of patients’ country, cumulative prednisolone dose at tn-1, immunosuppressant use at tn-1, time-adjusted SLEDAI score at tn-1, and for the presence of existing organ damage at tn-1.
*HRs adjusted patients age at tn-1, disease duration at tn-1, gross domestic product of patients’ country, cumulative prednisolone dose at tn-1, immunosuppressant use at tn-1, time-adjusted SLEDAI score at tn-1, and for the presence of existing organ damage at tn-1.
R. Kandane-Rathnayake: None; D. Milea: GSK, 3, 11; W. Louthrenoo: None; A. Hoi: Abbvie, 6, AstraZeneca, 5, Australian Rheumatology Association, 4, Eli Lilly, 6, EUSA Pharma (UK) Limited, 2, Limbic, 6, Moose Republic, 6, Novartis, 6; V. Golder: None; J. Cho: None; A. Lateef: None; S. Luo: None; Y. Wu: None; L. Hamijoyo: None; S. Sockalingam: AstraZeneca, 2, 12, Other - for attending meetings and/or travel, Pfizer, 2, 12, Other - for attending meetings and/or travel, ZP Therapeutics, 2; Z. Li: Abbott, 2, 9, Abbvie, 2, 9, BMS, 2, 9, Celgene, 2, 9, Eli Lilly, 2, 9, GSK, 2, 9, Janssen, 2, 9, MSD, 2, 9, Novartis, 2, 9, Pfizer, 2, 9, Roche, 2, 9, UCB Pharma, 2, 9; S. Navarra: Astellas, 6, AstraZeneca, 6, Biogen, 2, Boehringer-Ingelheim, 2, GSK, 6, Novartis, 6, Pfizer, 6; L. Zamora: None; M. Harigai: Astellas Pharma, 6, AstraZeneca, 6, GlaxoSmithKlein(GSK), 6, 12, Post marketing surveillence, Novartis, 5; Y. Katsumata: Asahi Kasei Pharma, 6, Astella, 6, AstraZeneca, 6, Chugai, 6, GlaxoSmithKlein(GSK), 6, Janssen, 6, Mitsubishi Tanabe Pharma Corporation, 6, Pfizer, 6; M. Chan: None; Y. Hao: None; Z. Zhang: None; S. O’Neill: None; F. Goldblatt: None; S. Oon: None; X. Xu: GSK, 3, 11; A. Amador Navarro Rojas: GSK, 3, 11; S. Bae: None; C. Lau: AstraZeneca, 6, 12, external expert for SLE sterring committee 11.2022; M. Nikpour: AstraZeneca, 2, 6, Boehringer-Ingelheim, 2, 6, GSK, 2, 6, Janssen Pharmaceuticals, 2, 5, 6; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5.



