Poster Session B
Osteoarthritis (OA) and related disorders
Session: (0859–0885) Osteoarthritis & Joint Biology – Basic Science Poster
0878: Synovial Fibroblasts Undergo a Phenotypic Shift from Early- to Late-stage Knee Osteoarthritis
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- JR
Jason Rockel, PhD
Schroeder Arthritis Institute, University Health Network
Toronto, ON, CanadaDisclosure information not submitted.
Abstract Poster Presenter(s)
Kabriya Thavaratnam1, Eric Gracey2, Anusha Ratneswaran1, Jason Rockel1, Shabana Vohra1, Chiara Pastrello3, Igor Jurisica4, starlee lively5, Sam Dupont6, Raja Rampersaud1, Nizar Mahomed7, Rajiv Gandhi8, Dirk Elewaut9 and Mohit Kapoor10, 1Schroeder Arthritis Institute, Toronto, ON, Canada, 2Ghent University Hospital, Gent, Belgium, 3Osteoarthritis Research Program, Division of Orthopaedic Surgery, Schroeder Arthritis Institute and Data Science Discovery Centre for Chronic diseases, Krembil Research Institute, Toronto, ON, Canada, 4Schroeder Arthritis Institute, Krembil Research Institute and Departments of Medical Biophysics and Computer Science and Faculty of Dentistry, University of Toronto and Institute of Neuroimmunology, Slovak Academy of Sciences, Bratislava, Slovakia, 5University Health Network, Toronto, ON, Canada, 6Ghent University Hospital, Ghent, Belgium, 7Division of Orthopaedics, Osteoarthritis Research Program, Schroeder Arthritis Institute, Krembil Research Institute, and Toronto Western Hospital, University Health Network, Toronto, ON, Canada, 8Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and Division of Orthopaedics, Department of Surgery, University of Toronto, Toronto, ON, Canada, 9Ghent University and VIB Center for Inflammation Research, Ghent, Belgium, 10Schroeder Arthritis Institute, University Health Network and Department of Surgery, University of Toronto, Toronto, ON, Canada
Background/Purpose: The synovium is a connective tissue that lines the joint capsule and is emerging as a key contributor to joint destruction during osteoarthritis (OA). During knee (K)OA pathogenesis, the synovium undergoes substantial changes including inflammation, cellular proliferation and fibrosis. However, the contributions of distinct synovial cell types, particularly fibroblasts (major synovial cells), to synovial pathologies in early and late-stages of KOA are unknown. To identify if distinct cell subtypes and their transcriptomic profiles exist in the synovium during distinct stages of OA disease severity, we used synovium from early (KL1) and late stages (KL3/4) of radiographic KOA and from a knee OA mouse model and subjected these samples to high throughput transcriptomic technologies such as single nucleus RNA sequencing (snRNAseq), bulk RNA sequencing, advanced bioinformatics and functional assays.
Methods: Synovia from early- (n=5; KL=1) and late-stage (n=4; KL=3/4) radiographic KOA patients were subjected to single nucleus (sn)RNAseq. Bulk RNA sequencing was performed in the synovium of n=6 early and n=8 late-stage radiographic KOA patients. Knee synovia isolated from naïve (control mice; n=4), 2 weeks (n=3) and 10 weeks (n=4) post-DMM surgery were also subjected to snRNAseq. Sequencing data was subjected to clustering analysis, differential expression analysis (to identify transcriptomic profiles), pathway analysis and upstream transcriptional regulator analyses.
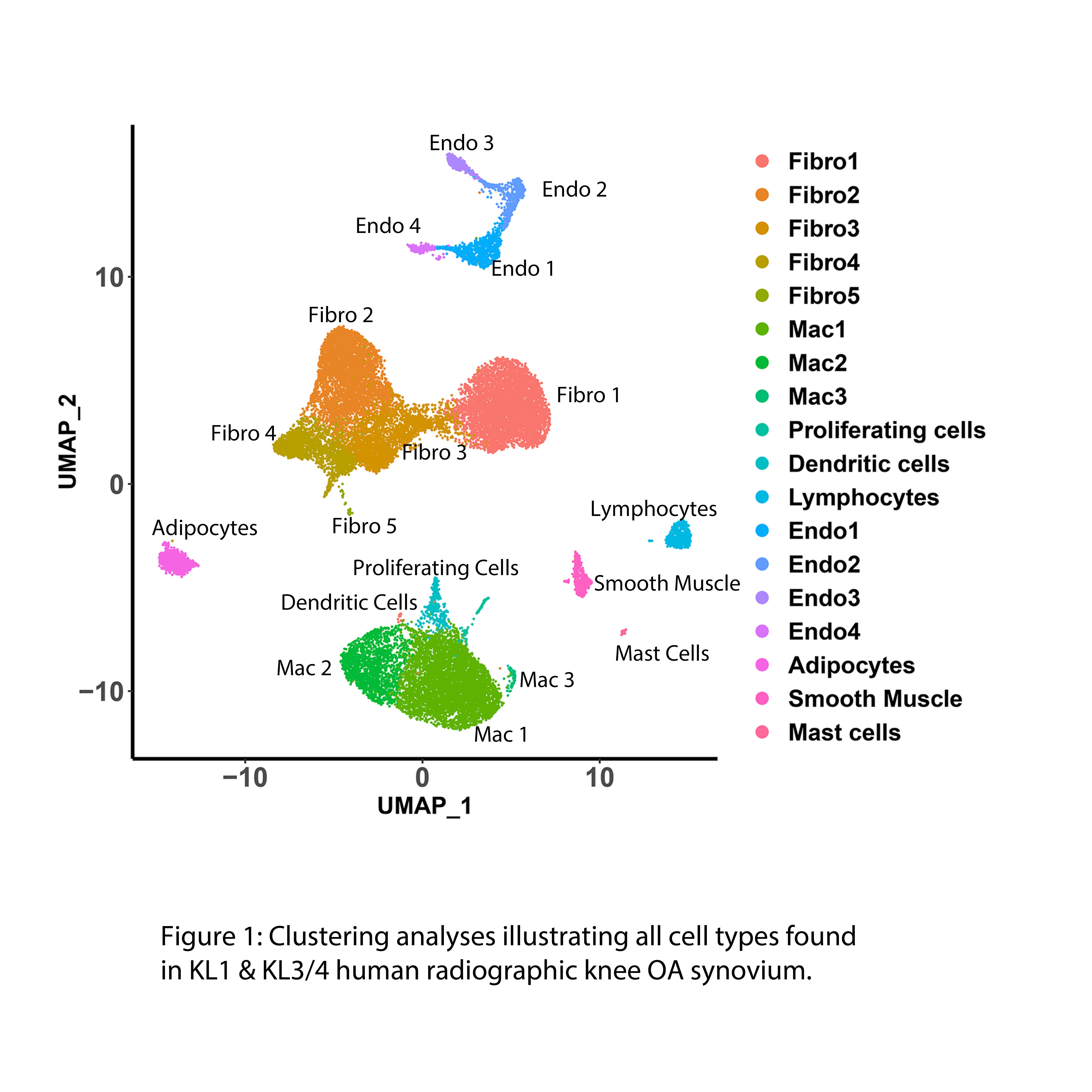
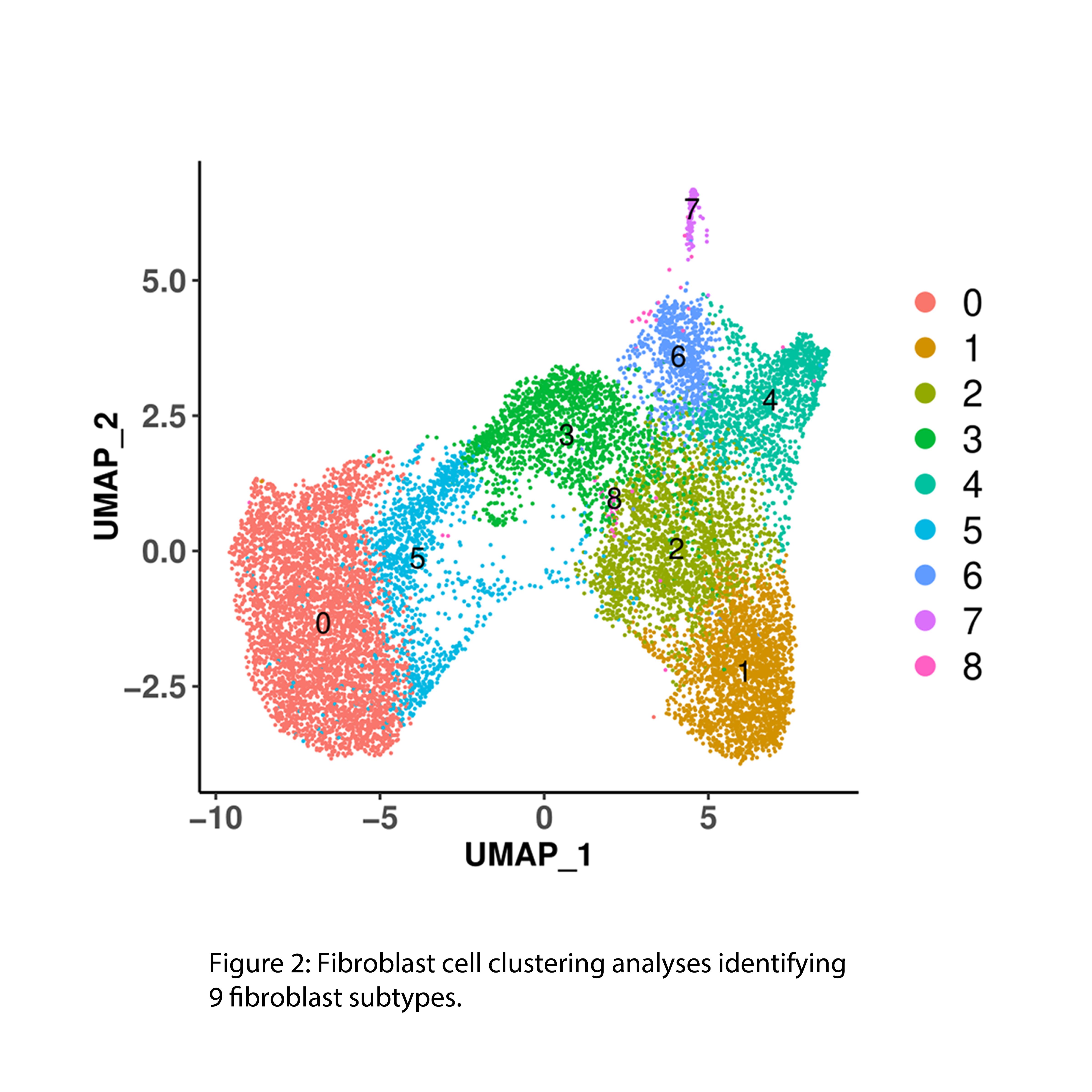
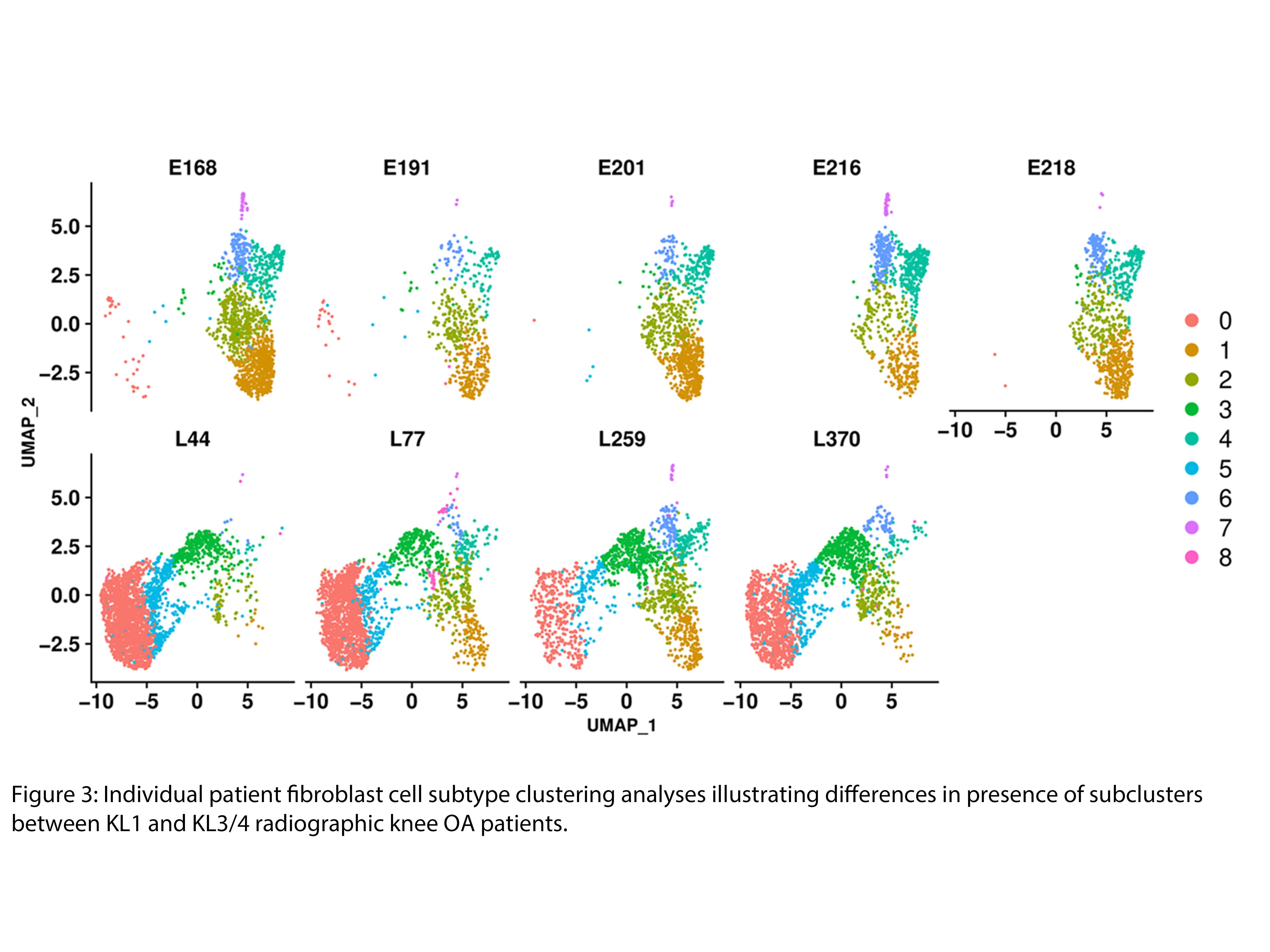
Results: Up to 50% of the cells from early and late-stage KOA human synovium were of fibroblast origin (Fig 1). Clustering analysis identified 8 distinct fibroblast subclusters in the KOA synovium (Fig 2). A phenotypic shift in fibroblast subsets was observed from early to late stages of KOA (fibroblast clusters 1, 2, 4 and 6 were predominantly associated with early-stage while fibroblast clusters 0, 3 and 5 were predominantly associated with late-stage OA) (Fig 3). Unique transcriptomic profiles were identified for each fibroblast subset, some being cell surface markers that were confirmed by immunohistochemistry to be differentially expressed in the synovium of early or late-stage KOA in vivo. Furthermore, pathway analyses suggest that the two major fibroblast subclusters, clusters 1 (early) and cluster 0 (late), may play crucial roles in ECM/fibrosis related pathways. Computational analysis has also identified putative upstream transcriptional regulators that may play key roles in ECM regulation and fibrosis. snRNAseq data of mouse synovium also identified some overlapping fibroblast clusters in human and mouse synovium. Specifically, late-stage cluster 0 identified in human snRNAseq analysis showed expansion in mouse synovium 10 weeks post DMM surgery compared to 2 week-DMM and naïve (control) synovium. Current efforts are focussed on targeting select transcription factor(s) using in vitro and in vivo gain and loss of function studies to identify their role in OA synovial pathology.
Conclusion: snRNAseq analysis has led us to identify distinct synovial fibroblast subsets with unique transcriptomic profiles in early and late-stages of KOA that may play a key role in driving OA synovial pathology.
K. Thavaratnam: None; E. Gracey: None; A. Ratneswaran: None; J. Rockel: None; S. Vohra: None; C. Pastrello: None; I. Jurisica: None; s. lively: None; S. Dupont: None; R. Rampersaud: None; N. Mahomed: AIC, 4, ARTHUR HEALTH CORP, 3; R. Gandhi: None; D. Elewaut: AbbVie/Abbott, 6, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 2, galapagos, 5, Janssen, 6; M. Kapoor: None.
Background/Purpose: The synovium is a connective tissue that lines the joint capsule and is emerging as a key contributor to joint destruction during osteoarthritis (OA). During knee (K)OA pathogenesis, the synovium undergoes substantial changes including inflammation, cellular proliferation and fibrosis. However, the contributions of distinct synovial cell types, particularly fibroblasts (major synovial cells), to synovial pathologies in early and late-stages of KOA are unknown. To identify if distinct cell subtypes and their transcriptomic profiles exist in the synovium during distinct stages of OA disease severity, we used synovium from early (KL1) and late stages (KL3/4) of radiographic KOA and from a knee OA mouse model and subjected these samples to high throughput transcriptomic technologies such as single nucleus RNA sequencing (snRNAseq), bulk RNA sequencing, advanced bioinformatics and functional assays.
Methods: Synovia from early- (n=5; KL=1) and late-stage (n=4; KL=3/4) radiographic KOA patients were subjected to single nucleus (sn)RNAseq. Bulk RNA sequencing was performed in the synovium of n=6 early and n=8 late-stage radiographic KOA patients. Knee synovia isolated from naïve (control mice; n=4), 2 weeks (n=3) and 10 weeks (n=4) post-DMM surgery were also subjected to snRNAseq. Sequencing data was subjected to clustering analysis, differential expression analysis (to identify transcriptomic profiles), pathway analysis and upstream transcriptional regulator analyses.
Results: Up to 50% of the cells from early and late-stage KOA human synovium were of fibroblast origin (Fig 1). Clustering analysis identified 8 distinct fibroblast subclusters in the KOA synovium (Fig 2). A phenotypic shift in fibroblast subsets was observed from early to late stages of KOA (fibroblast clusters 1, 2, 4 and 6 were predominantly associated with early-stage while fibroblast clusters 0, 3 and 5 were predominantly associated with late-stage OA) (Fig 3). Unique transcriptomic profiles were identified for each fibroblast subset, some being cell surface markers that were confirmed by immunohistochemistry to be differentially expressed in the synovium of early or late-stage KOA in vivo. Furthermore, pathway analyses suggest that the two major fibroblast subclusters, clusters 1 (early) and cluster 0 (late), may play crucial roles in ECM/fibrosis related pathways. Computational analysis has also identified putative upstream transcriptional regulators that may play key roles in ECM regulation and fibrosis. snRNAseq data of mouse synovium also identified some overlapping fibroblast clusters in human and mouse synovium. Specifically, late-stage cluster 0 identified in human snRNAseq analysis showed expansion in mouse synovium 10 weeks post DMM surgery compared to 2 week-DMM and naïve (control) synovium. Current efforts are focussed on targeting select transcription factor(s) using in vitro and in vivo gain and loss of function studies to identify their role in OA synovial pathology.
Conclusion: snRNAseq analysis has led us to identify distinct synovial fibroblast subsets with unique transcriptomic profiles in early and late-stages of KOA that may play a key role in driving OA synovial pathology.



K. Thavaratnam: None; E. Gracey: None; A. Ratneswaran: None; J. Rockel: None; S. Vohra: None; C. Pastrello: None; I. Jurisica: None; s. lively: None; S. Dupont: None; R. Rampersaud: None; N. Mahomed: AIC, 4, ARTHUR HEALTH CORP, 3; R. Gandhi: None; D. Elewaut: AbbVie/Abbott, 6, Bristol-Myers Squibb(BMS), 5, Eli Lilly, 2, galapagos, 5, Janssen, 6; M. Kapoor: None.



