Poster Session B
Rheumatoid arthritis (RA)
Session: (1264–1307) RA – Diagnosis, Manifestations, and Outcomes Poster II
1297: COVID-19 Vaccination-related Delayed Adverse Events Among Patients with Rheumatoid Arthritis: Results from the COVAD Study
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- MD
Mrinalini Dey, MRCP, MA, MBBCH
Centre for Rheumatic Diseases, King's College London
London, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Mrinalini Dey1, Bohdana Doskaliuk2, Ioannis Parodis3, Julius Lindblom3, Chris Wincup4, Mrudula Joshi5, Dey Dzifa6, Esha Kadam7, Parikshit Sen8, Samuel Shinjo9, Arvind Nune10, Nelly Ziade11, Yi-Ming Chen12, Lisa Traboco13, CARLOS ENRIQUE TORO GUTIERREZ14, COVAD Study Group15, Rohit Aggarwal16, Vikas Agarwal17, Latika Gupta18 and Elena Nikiphorou19, 1Queen Elizabeth Hospital, London, United Kingdom; University of Liverpool, Liverpool, United Kingdom, 2SHEI \"Ivano-Frankivsk national medical university\", Ivano-Frankivsk, Ukraine, 3Karolinska Institutet, Stockholm, Sweden, 4King's College Hospital, London, United Kingdom, 5Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, India, 6Department of Medicine and Therapeutics, University of Ghana School of Medicine and Dentistry, College of Health Sciences, Korle-Bu, Accra, Ghana, 7Seth Gordhandhas Sunderdas Medical College and King Edwards Memorial Hospital, Mumbai, India, 8Maulana Azad Medical College, 2-Bahadurshah Zafar Marg, New Delhi, Delhi-110002, India., Dalhi, India, 9Faculdade de Medicina FMUSP, Universidade de Sao Paulo, São Paulo, Brazil, 10Southport & Ormskirk NHS Foundation Trust, Liverpool, United Kingdom, 11Saint-Joseph University, Beirut, Lebanon, 12Taichung Veterans General Hospital, Taichung, Taiwan, 13University of the Philippines - Manila, St Luke's Medical Center - Bonifacio Global City, Paranaque, Manila, Philippines, 14Centro de Estudios de Reumatología y Dermatología SAS, Cali, Colombia, 15-, -, 16University of Pittsburgh, Pittsburgh, PA, 17Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, India, 18Royal Wolverhampton Trust, Wolverhampton/University of Manchester, United Kingdom, 19King's College London, London, United Kingdom
Background/Purpose: COVID-19 vaccines have been proven to be safe in the healthy population. Data on longer-term AEs in people with autoimmune diseases (AIDs), including rheumatoid arthritis (RA) are lacking. COVID-19 vaccination-related AEs in patients with RA, rheumatic (rAIDs), and non-rheumatic AIDs (nrAIDs) and healthy controls (HC) greater than seven days post-vaccination were assessed in the COVID-19 Vaccination in Autoimmune Diseases (COVAD)-2 study.
Methods: The COVAD-2 study group comprised 157 collaborators across 106 countries. The study was conducted between February and June 2022. An online survey captured self-reported data related to COVID-19 vaccination-related AEs in RA, AIDs, and HCs. rAIDs included connective tissue diseases, inflammatory myopathies and inflammatory arthritis. nrAIDs comprised inflammatory bowel disease, multiple sclerosis etc. We compared COVID-19 vaccination-related delayed AEs among RA, other rAIDs, nrAIDs and HCs, adjusting for age, gender and ethnicity, using multivariable binary regression. Statistically significant results are reported.
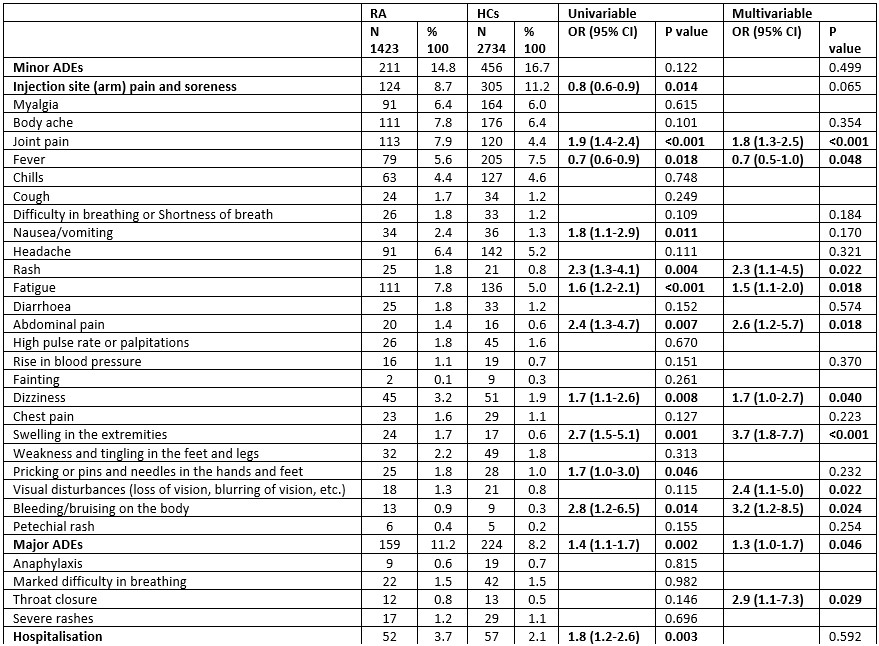
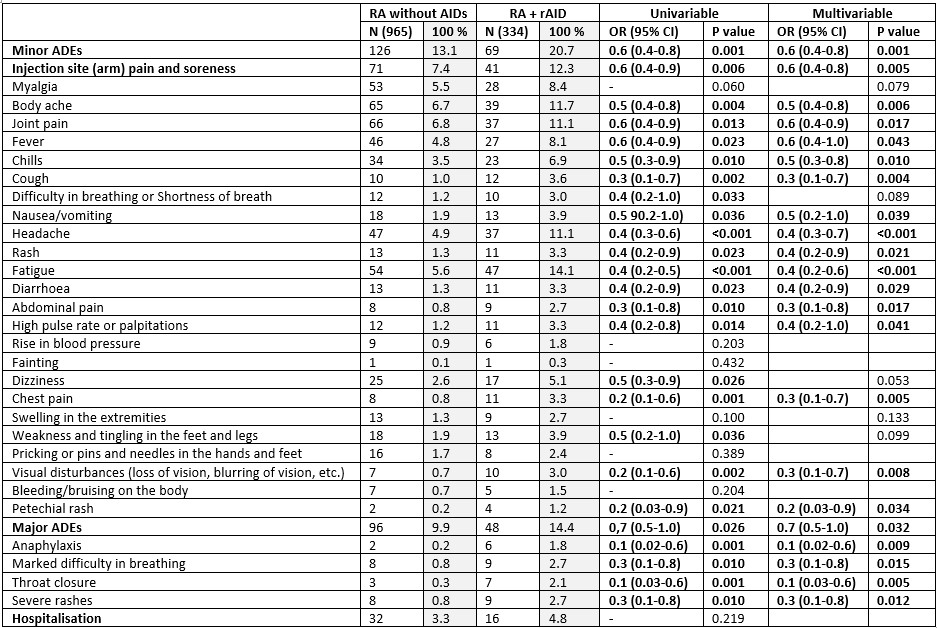
Results: Among 7203 participants, 1423 (19.7%) RA, 2620 (36.4%) rAIDs, 426 (5.9%) nrAIDs and 2734 (38%) HCs were included from a total of 17612 respondents, with 75% female and 42.2% Caucasian (Table 1). People with RA were older [median age 51 (40-62); rAIDs, 48 (37-59); nrAIDs 43 (34-53); HC 38 (30-49)]. When compared to HCs, people with RA reported higher overall major AEs in the multivariable analyses [OR 1.3 (1.0-1.7)], especially throat closure [OR 2.9 (1.1-7.3)], and increased number of several minor AEs (Table 2). People with RA had fewer reported episodes of fever [OR 0.7 (0.5-1.0)]. When compared to rAIDS, people with RA had fewer episodes of rash [OR 0.6 (0.4-1.0)], and when compared to nrAIDs, had increased reported injection site pain [OR 1.7 (1.0-2.6)], myalgia [OR 1.9 (1.1-3.4)], body ache [OR 2.2 (1.3-3.7)], joint pain [OR 2.7 (1.5-4.8)], fever [OR 1.8 (1.0-3.3)] and swelling of extremities [OR 4.9 (1.1-21.4)]. ChadOx1 nCOV-19 (Oxford/ AstraZeneca) led to significantly increased minor AEs in the RA group [OR 1.9 (1.4-2.6)], compared to other vaccines, while the Moderna vaccination was associated with increased hospitalisation in people with RA [OR 2.4 (1.3-4.3)]. People with active RA had increased major AEs [OR 1.8 (1.1 – 3.0)] and hospitalisation [OR 4.1 (1.3 – 13.3)], compared to inactive RA. RA patients without autoimmune comorbidities had significantly fewer major and minor AEs compared to those with other rAIDs (Table 3). People with RA and mental health diagnoses had increased reported chills [OR 1.8 (1.1 – 3.0)] and chest pain [OR 2.5 (1.1 – 6.0)]. Decreased incidence of hospitalisation was seen in patients taking methotrexate [OR 0.5 (0.3-0.9)] or TNF inhibitors [OR 0.1 (0.02-0.9)] compared to hydroxychloroquine, sulfasalazine and leflunomide.
Conclusion: COVID-19 vaccination is safe with minimal to no risks of delayed AEs in patients with RA compared to HCs, and fewer compared to other rAIDs. Active RA and presence of co-existent rAIDs were both associated with increased risk of delayed AEs.
.jpg)
M. Dey: None; B. Doskaliuk: None; I. Parodis: Amgen, 5, 6, AstraZeneca, 5, 6, Aurinia Pharmaceuticals, 5, 6, Bristol-Myers Squibb(BMS), 5, 6, Elli Lilly and Company, 5, 6, F. Hoffmann-La Roche AG, 5, 6, Gilead Sciences, 5, 6, GSK, 5, 6, Janssen Pharmaceuticals, 5, 6, Novartis, 5, 6, Otsuka Pharmaceutical, 5, 6; J. Lindblom: None; C. Wincup: None; M. Joshi: None; D. Dzifa: Roche, 6; E. Kadam: None; P. Sen: None; S. Shinjo: None; A. Nune: None; N. Ziade: Abbvie, 6, Boehringer-Ingelheim, 6, Eli Lilly, 6, Janssen, 6, Newbridge, 6, Novartis, 6, Pfizer, 6, Pierre Fabre, 6, Roche, 6, sanofi, 6; Y. Chen: None; L. Traboco: None; C. TORO GUTIERREZ: AbbVie/Abbott, 6, Boehringer-Ingelheim, 6, Janssen, 6; C. Study Group: None; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2; V. Agarwal: None; L. Gupta: None; E. Nikiphorou: AbbVie/Abbott, 6, Celltrion, 6, Eli Lilly, 6, fresenius, 6, Galapagos, 6, Gilead, 1, 6, Pfizer, 6, Sanofi, 6.
Background/Purpose: COVID-19 vaccines have been proven to be safe in the healthy population. Data on longer-term AEs in people with autoimmune diseases (AIDs), including rheumatoid arthritis (RA) are lacking. COVID-19 vaccination-related AEs in patients with RA, rheumatic (rAIDs), and non-rheumatic AIDs (nrAIDs) and healthy controls (HC) greater than seven days post-vaccination were assessed in the COVID-19 Vaccination in Autoimmune Diseases (COVAD)-2 study.
Methods: The COVAD-2 study group comprised 157 collaborators across 106 countries. The study was conducted between February and June 2022. An online survey captured self-reported data related to COVID-19 vaccination-related AEs in RA, AIDs, and HCs. rAIDs included connective tissue diseases, inflammatory myopathies and inflammatory arthritis. nrAIDs comprised inflammatory bowel disease, multiple sclerosis etc. We compared COVID-19 vaccination-related delayed AEs among RA, other rAIDs, nrAIDs and HCs, adjusting for age, gender and ethnicity, using multivariable binary regression. Statistically significant results are reported.
Results: Among 7203 participants, 1423 (19.7%) RA, 2620 (36.4%) rAIDs, 426 (5.9%) nrAIDs and 2734 (38%) HCs were included from a total of 17612 respondents, with 75% female and 42.2% Caucasian (Table 1). People with RA were older [median age 51 (40-62); rAIDs, 48 (37-59); nrAIDs 43 (34-53); HC 38 (30-49)]. When compared to HCs, people with RA reported higher overall major AEs in the multivariable analyses [OR 1.3 (1.0-1.7)], especially throat closure [OR 2.9 (1.1-7.3)], and increased number of several minor AEs (Table 2). People with RA had fewer reported episodes of fever [OR 0.7 (0.5-1.0)]. When compared to rAIDS, people with RA had fewer episodes of rash [OR 0.6 (0.4-1.0)], and when compared to nrAIDs, had increased reported injection site pain [OR 1.7 (1.0-2.6)], myalgia [OR 1.9 (1.1-3.4)], body ache [OR 2.2 (1.3-3.7)], joint pain [OR 2.7 (1.5-4.8)], fever [OR 1.8 (1.0-3.3)] and swelling of extremities [OR 4.9 (1.1-21.4)]. ChadOx1 nCOV-19 (Oxford/ AstraZeneca) led to significantly increased minor AEs in the RA group [OR 1.9 (1.4-2.6)], compared to other vaccines, while the Moderna vaccination was associated with increased hospitalisation in people with RA [OR 2.4 (1.3-4.3)]. People with active RA had increased major AEs [OR 1.8 (1.1 – 3.0)] and hospitalisation [OR 4.1 (1.3 – 13.3)], compared to inactive RA. RA patients without autoimmune comorbidities had significantly fewer major and minor AEs compared to those with other rAIDs (Table 3). People with RA and mental health diagnoses had increased reported chills [OR 1.8 (1.1 – 3.0)] and chest pain [OR 2.5 (1.1 – 6.0)]. Decreased incidence of hospitalisation was seen in patients taking methotrexate [OR 0.5 (0.3-0.9)] or TNF inhibitors [OR 0.1 (0.02-0.9)] compared to hydroxychloroquine, sulfasalazine and leflunomide.
Conclusion: COVID-19 vaccination is safe with minimal to no risks of delayed AEs in patients with RA compared to HCs, and fewer compared to other rAIDs. Active RA and presence of co-existent rAIDs were both associated with increased risk of delayed AEs.
.jpg)
Table 1: Socio-demographic and vaccination data of survey respondents. RA: rheumatoid arthritis; rAID: rheumatic autoimmune disease; nrAID: non-rheumatic autoimmune disease; HC: health controls.

Table 2: Effects of COVID-19 vaccination in patients with rheumatoid arthritis (RA) vs healthy controls (HCs). Factors included as covariates in multivariable binary logistic regression analysis included age, sex, ethnicity.

Table 3: Effects of COVID-19 vaccination in patients with rheumatoid arthritis (RA) and no autoimmune comorbidities vs RA with rheumatic autoimmune disease (rAIDs). Factors included as covariates in multivariable binary logistic regression analysis included age, sex, ethnicity.
M. Dey: None; B. Doskaliuk: None; I. Parodis: Amgen, 5, 6, AstraZeneca, 5, 6, Aurinia Pharmaceuticals, 5, 6, Bristol-Myers Squibb(BMS), 5, 6, Elli Lilly and Company, 5, 6, F. Hoffmann-La Roche AG, 5, 6, Gilead Sciences, 5, 6, GSK, 5, 6, Janssen Pharmaceuticals, 5, 6, Novartis, 5, 6, Otsuka Pharmaceutical, 5, 6; J. Lindblom: None; C. Wincup: None; M. Joshi: None; D. Dzifa: Roche, 6; E. Kadam: None; P. Sen: None; S. Shinjo: None; A. Nune: None; N. Ziade: Abbvie, 6, Boehringer-Ingelheim, 6, Eli Lilly, 6, Janssen, 6, Newbridge, 6, Novartis, 6, Pfizer, 6, Pierre Fabre, 6, Roche, 6, sanofi, 6; Y. Chen: None; L. Traboco: None; C. TORO GUTIERREZ: AbbVie/Abbott, 6, Boehringer-Ingelheim, 6, Janssen, 6; C. Study Group: None; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2; V. Agarwal: None; L. Gupta: None; E. Nikiphorou: AbbVie/Abbott, 6, Celltrion, 6, Eli Lilly, 6, fresenius, 6, Galapagos, 6, Gilead, 1, 6, Pfizer, 6, Sanofi, 6.



