Poster Session A
Infection-related rheumatic syndromes
Session: (0196–0228) Infection-related Rheumatic Disease Poster
0225: Breakthrough SARS-Cov-2 Infection and Disease Flares in Patients with Rheumatoid Arthritis: Result from COVAD E-Survey Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- CT
CARLOS ENRIQUE TORO GUTIERREZ, MD, MSc
Centro de Estudios de Reumatología y Dermatología SAS
Cali, ColombiaDisclosure information not submitted.
Abstract Poster Presenter(s)
Yi-Ming Chen1, Jun-Pen Chen2, Chi-Wei Tseng2, CARLOS ENRIQUE TORO GUTIERREZ3, Elena Nikiphorou4, Ai Lyn Tan5, Arvind Nune6, Esha Kadam7, Masataka Kuwana8, Jessica Day9, Sreoshy Saha10, Tsvetelina Velikova11, James Lilleker12, CARLO VINICIO CABALLERO13, Parikshit Sen14, Hector Chinoy15, Rohit Aggarwal16, Vikas Agarwal17 and Latika Gupta18, 1Taichung Veterans General Hospital, Taichung, Taiwan, 2Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung City, Taiwan, 3Centro de Estudios de Reumatología y Dermatología SAS, Cali, Colombia, 4King's College London, London, United Kingdom, 5University of Leeds, Leeds, United Kingdom, 6Southport & Ormskirk NHS Foundation Trust, Liverpool, United Kingdom, 7Seth Gordhandhas Sunderdas Medical College and King Edwards Memorial Hospital, Mumbai, India, 8Nippon Medical School Graduate School of Medicine, Tokyo, Japan, 9Walter and Eliza Hall Institute, Melbourne, Australia, 10Mymensingh Medical College, Faridpur, Bangladesh, 11Medical Faculty, Sofia University St. Kliment Ohridski, Sofia, Bulgaria, 12Centre for Musculoskeletal Research, Division of Musculoskeletal and Dermatological Sciences, School of Biological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK; Manchester Centre for Clinical Neurosciences, Salford Royal NHS Foundation Trust, Salford, UK. Orcid ID: 0000-0002-9230-4137., Manchester, United Kingdom, 13REUMACARIBE IPS, Barranquilla, Colombia, 14Maulana Azad Medical College, 2-Bahadurshah Zafar Marg, New Delhi, Delhi-110002, India., Dalhi, India, 15The University of Manchester, Sale, United Kingdom, 16University of Pittsburgh, Pittsburgh, PA, 17Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS), Lucknow, India, 18Royal Wolverhampton Trust, Wolverhampton/University of Manchester, United Kingdom
Background/Purpose: Existing studies have shown that disease activity of patients with rheumatoid arthritis (RA) may surge after COVID-19 infection. However, factors associated with disease flares remain unknown. This study aimed to identify factors associated with breakthrough infection and disease flares in patients with RA following COVID-19 infection.
Methods: We selected patients with RA from an online e-survey data from the COVAD study. Demographic data, patient reported outcomes, comorbidities and pharmacologic treatments were extracted from the database. Disease flare-up was derived from the e-survey database. Factors associated with disease flare-up were determined by multivariable logistic regression analysis.
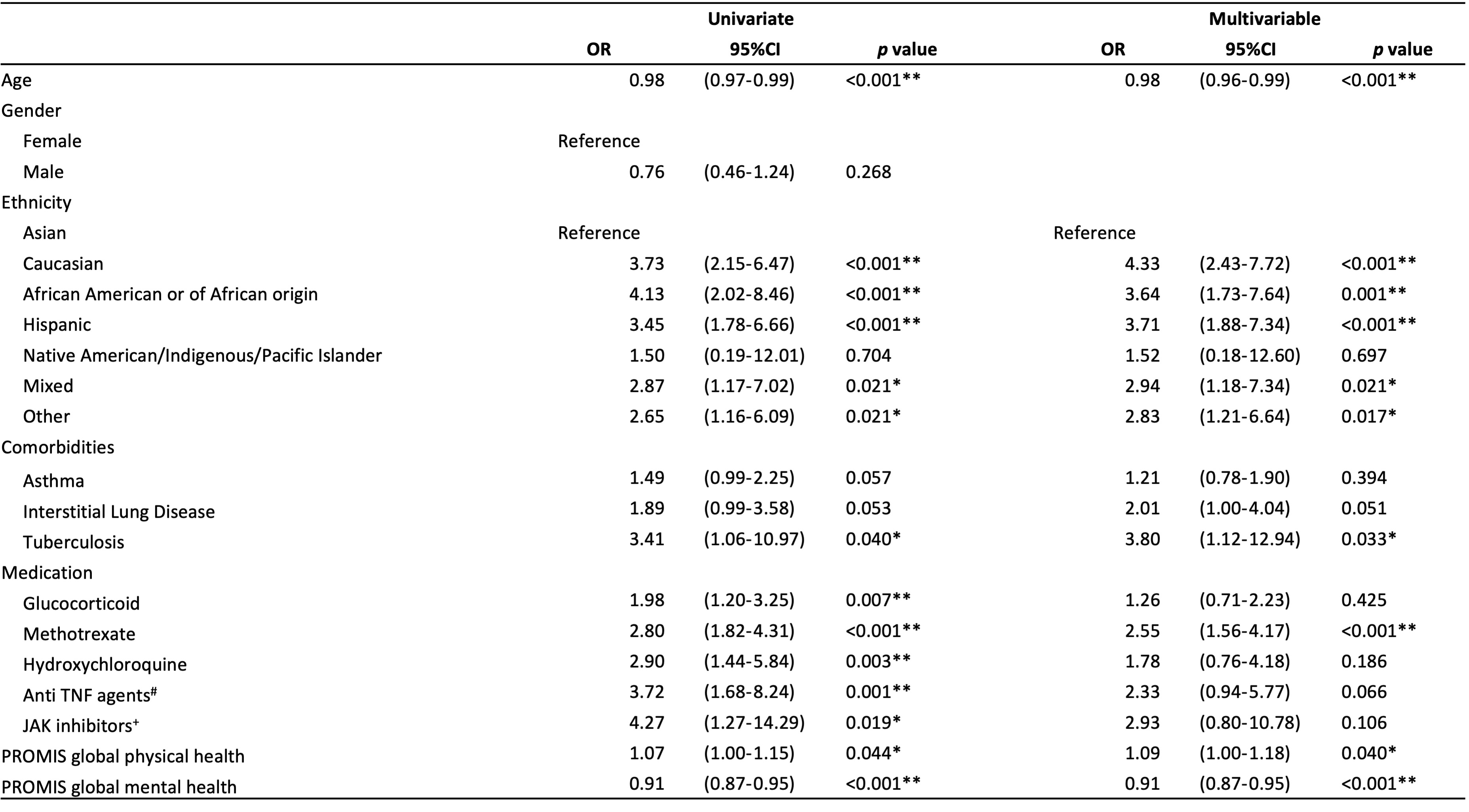
Results: In total, 1928 patients with RA were extracted from the COVAD database. Older age, Caucasian ethnicity, comorbidities with chronic kidney disease and asthma, were associated with COVID-19 breakthrough infection. Moreover, younger age (odds ratio, OR: 0.98, 95% CI: 0.96 – 0.99, p< 0.001), ethnicity other than Asian, tuberculosis (OR: 3.80, 95% CI: 1.12 – 12.94, p=0.033), treatment with methotrexate (OR: 2.55, 95% CI: 1.56 – 4.17, p< 0.001), poor global physical health (OR: 1.07, 95% CI: 1.00 –1.15, p=0.044) and mental health (OR: 0.91, 95% CI: 0.87 –0.95, p< 0.001) were independent factors associated with disease flares in patients with RA.
Conclusion: Our study highlights the necessity for rheumatologists to recognize potential predictors of RA flare-up following COVID-19 infection. Proactive strategies are recommended for managing high-risk RA patients.
.jpg)
Y. Chen: None; J. Chen: None; C. Tseng: None; C. TORO GUTIERREZ: AbbVie/Abbott, 6, Boehringer-Ingelheim, 6, Janssen, 6; E. Nikiphorou: AbbVie/Abbott, 6, Celltrion, 6, Eli Lilly, 6, fresenius, 6, Galapagos, 6, Gilead, 1, 6, Pfizer, 6, Sanofi, 6; A. Tan: Abbvie, 1, 6, Gilead, 6, Janssen, 6, Lilly, 6, Novartis, 6, Pfizer, 6, UCB, 6; A. Nune: None; E. Kadam: None; M. Kuwana: AbbVie/Abbott, 6, Asahi-Kasei, 5, 6, Astellas, 6, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, 6, Chugai, 2, 5, 6, Corbus, 2, Eisai, 6, GlaxoSmithKlein(GSK), 2, Horizon, 2, Janssen, 6, Kissei, 2, MBL, 2, 5, Mitsubishi Tanabe, 2, 5, 6, Mochida, 2, 6, Nippon Shinyaku, 6, Ono, 5, 6; J. Day: CSL limited, 5; S. Saha: None; T. Velikova: AstraZeneca, 6, Pfizer, 6; J. Lilleker: None; C. CABALLERO: None; P. Sen: None; H. Chinoy: AstraZeneca, 1, Biogen, 2, Eli Lilly, 5, GlaxoSmithKlein(GSK), 6, Novartis, 2, Orphazyme, 2, Pfizer, 1, UCB, 6; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2; V. Agarwal: None; L. Gupta: None.
Background/Purpose: Existing studies have shown that disease activity of patients with rheumatoid arthritis (RA) may surge after COVID-19 infection. However, factors associated with disease flares remain unknown. This study aimed to identify factors associated with breakthrough infection and disease flares in patients with RA following COVID-19 infection.
Methods: We selected patients with RA from an online e-survey data from the COVAD study. Demographic data, patient reported outcomes, comorbidities and pharmacologic treatments were extracted from the database. Disease flare-up was derived from the e-survey database. Factors associated with disease flare-up were determined by multivariable logistic regression analysis.
Results: In total, 1928 patients with RA were extracted from the COVAD database. Older age, Caucasian ethnicity, comorbidities with chronic kidney disease and asthma, were associated with COVID-19 breakthrough infection. Moreover, younger age (odds ratio, OR: 0.98, 95% CI: 0.96 – 0.99, p< 0.001), ethnicity other than Asian, tuberculosis (OR: 3.80, 95% CI: 1.12 – 12.94, p=0.033), treatment with methotrexate (OR: 2.55, 95% CI: 1.56 – 4.17, p< 0.001), poor global physical health (OR: 1.07, 95% CI: 1.00 –1.15, p=0.044) and mental health (OR: 0.91, 95% CI: 0.87 –0.95, p< 0.001) were independent factors associated with disease flares in patients with RA.
Conclusion: Our study highlights the necessity for rheumatologists to recognize potential predictors of RA flare-up following COVID-19 infection. Proactive strategies are recommended for managing high-risk RA patients.
.jpg)
Table 1. Factors associated with breakthrough COVID-19 infection in patients with RA
By Chi-square test or ANOVA test. *p<0.05, **p<0.01
#infliximab, adalimumab, certolizumab, golimumab, etanercept
+tofacitinib, baricitinib, upadacitinib
By Chi-square test or ANOVA test. *p<0.05, **p<0.01
#infliximab, adalimumab, certolizumab, golimumab, etanercept
+tofacitinib, baricitinib, upadacitinib

Table 2. Factors associated with disease flare in patients with RA after COVID-19 infection.
By Logistic regression. *p<0.05, **p<0.01.
#infliximab, adalimumab, certolizumab, golimumab, etanercept
+tofacitinib, baricitinib, upadacitinib
By Logistic regression. *p<0.05, **p<0.01.
#infliximab, adalimumab, certolizumab, golimumab, etanercept
+tofacitinib, baricitinib, upadacitinib
Y. Chen: None; J. Chen: None; C. Tseng: None; C. TORO GUTIERREZ: AbbVie/Abbott, 6, Boehringer-Ingelheim, 6, Janssen, 6; E. Nikiphorou: AbbVie/Abbott, 6, Celltrion, 6, Eli Lilly, 6, fresenius, 6, Galapagos, 6, Gilead, 1, 6, Pfizer, 6, Sanofi, 6; A. Tan: Abbvie, 1, 6, Gilead, 6, Janssen, 6, Lilly, 6, Novartis, 6, Pfizer, 6, UCB, 6; A. Nune: None; E. Kadam: None; M. Kuwana: AbbVie/Abbott, 6, Asahi-Kasei, 5, 6, Astellas, 6, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, 6, Chugai, 2, 5, 6, Corbus, 2, Eisai, 6, GlaxoSmithKlein(GSK), 2, Horizon, 2, Janssen, 6, Kissei, 2, MBL, 2, 5, Mitsubishi Tanabe, 2, 5, 6, Mochida, 2, 6, Nippon Shinyaku, 6, Ono, 5, 6; J. Day: CSL limited, 5; S. Saha: None; T. Velikova: AstraZeneca, 6, Pfizer, 6; J. Lilleker: None; C. CABALLERO: None; P. Sen: None; H. Chinoy: AstraZeneca, 1, Biogen, 2, Eli Lilly, 5, GlaxoSmithKlein(GSK), 6, Novartis, 2, Orphazyme, 2, Pfizer, 1, UCB, 6; R. Aggarwal: Actigraph, 2, Alexion, 2, ANI Pharmaceuticals, 2, Argenx, 2, AstraZeneca, 2, Boehringer-Ingelheim, 2, 5, Bristol-Myers Squibb(BMS), 2, 5, CabalettaBio, 2, Capella Bioscience, 2, Corbus, 2, CSL Behring, 2, EMD Serono, 2, 5, Galapagos, 2, Horizon Therapeutics, 2, I-Cell, 2, Janssen, 2, 5, Kezar, 2, Kyverna, 2, Mallinckrodt, 5, Merck, 2, Octapharma, 2, Pfizer, 2, 5, Q32, 5, Roivant, 2, Sanofi, 2, Teva, 2; V. Agarwal: None; L. Gupta: None.



