Poster Session A
Infection-related rheumatic syndromes
Session: (0196–0228) Infection-related Rheumatic Disease Poster
0209: Safety of Bivalent SARS-CoV-2 Vaccines as a Second Booster Dose in Arthritis Patients on Immunosuppressive Therapies
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- HÃ
Abstract Poster Presenter(s)
Hilde Ørbo1, Ingrid Jyssum1, Anne Therese Tveter1, Ingrid Christensen1, Joseph Sexton1, Kristin Hammersbøen Bjørlykke2, Grete B. Kro3, Tore Kvien1, Gunnveig Grødeland4, Ludvig A. Munthe5, Siri Mjaaland6, John Torgils Vaage5, Espen Haavardsholm1, Kristin Kaasen Jørgensen2, Sella Provan7, Silje Watterdal Syversen1 and Guro Goll1, 1Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, 2Department of Gastroenterology, Akershus University Hospital, Oslo, Norway, 3Department of Microbiology, Oslo University Hospital, Oslo, Norway, 4Department of Immunology, Oslo University Hospital, Oslo, Norway, 5Department of Immunology, Oslo University Hospital, Oslo, Norway, 6Norwegian Institute of Public Health, Oslo, Norway, 7Diakonhjemmet Hospital, Oslo, Norway
Background/Purpose: Safety and efficacy of updated bivalent vaccines, containing both the original vaccine variant of SARS-CoV-2 Spike and either Omicron variants BA.1 or BA.4/5, are of particular interest in arthritis patients on immunosuppressive therapies. With the continuous emergence of new viral variants, it is important to evaluate whether updated vaccines induce more adverse events in this patient group. The objective of this study was examine if a second booster dose with updated bivalent vaccine increases the risk of adverse events, compared to the first booster dose with monovalent vaccines.
Methods: The prospective Nor-vaC study investigates vaccine responses in patients with immune mediated inflammatory diseases using immunosuppressive therapies (1). The present analyses included arthritis patients who received two booster doses. Patients received available vaccines according to the Norwegian vaccination program. The current recommendation in the Norwegian arthritis population is a three-dose primary vaccination series followed by two booster doses. Adverse events following vaccines doses were self-reported through questionnaires. Adverse events following the first (monovalent) and second (bivalent) booster were compared with McNemar’s test.
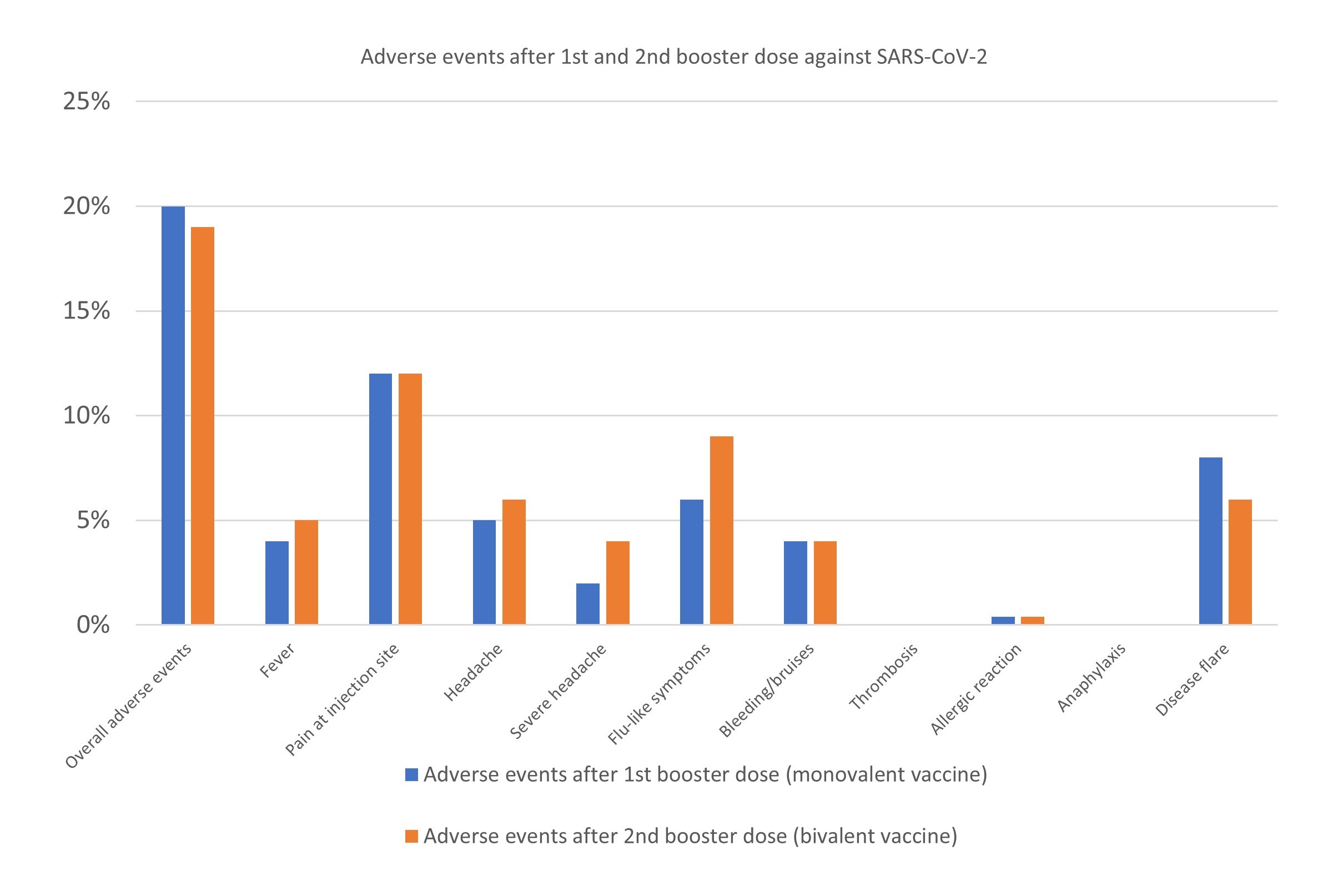
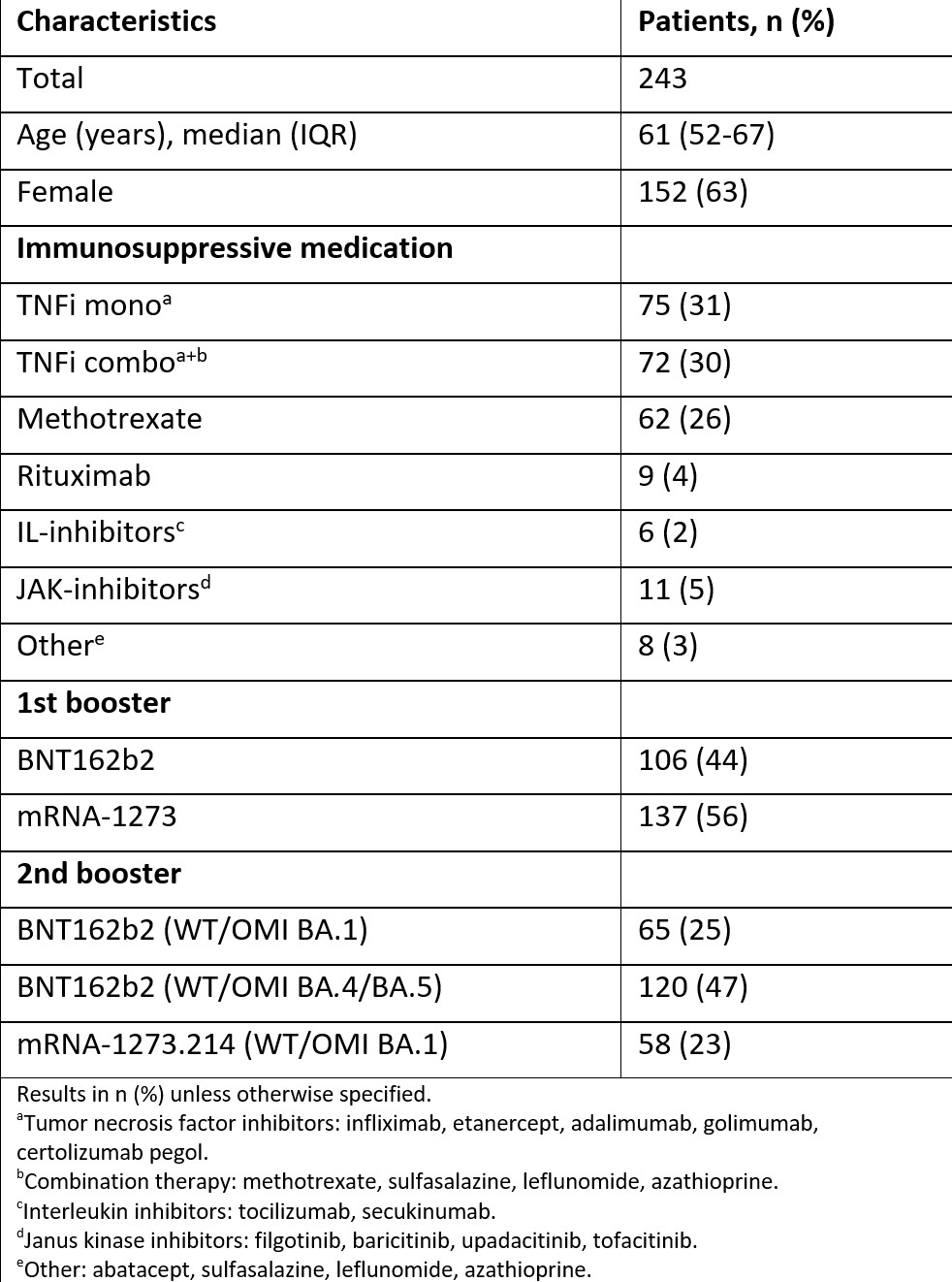
Results: Between 7th of July 2021 and 6th of December 2022 a total of 243 arthritis patients (127 rheumatoid arthritis, 65 psoriatic arthritis, 51 spondyloarthritis) on immunosuppressive therapies (Table) received a first, monovalent (BNT162b2, mRNA-1273) and a second, bivalent booster dose (BNT162b2 (WT/OMI BA.1), mRNA-1273.214, BNT162b2 (WT/OMI BA.4/BA.5)). Adverse events were recorded within two weeks in all patients (Figure). In total, 45 vs 49 (19% vs 20 %) patients reported any adverse event after a second, bivalent booster dose, compared to the first, monovalent booster, respectively. There was no significant difference in adverse events overall (p= 0.57). The most common adverse events after the second booster were pain at injection site (12 %), flu-like symptoms (9 %) and headache (6 %). No new safety signals emerged. A total of 15 (6 %) patients reported a disease flare after receiving the second, bivalent booster, compared to 21 (8 %) after the first, monovalent booster.
Conclusion: There was no difference in adverse events between the monovalent, first booster, and the bivalent, second booster, indicating that bivalent vaccines are safe in this patient group.
Reference:
1. Syversen S.W. et al Arthritis Rheumatol 2022
H. Ørbo: None; I. Jyssum: None; A. Tveter: None; I. Christensen: None; J. Sexton: None; K. Bjørlykke: Janssen-Cilag, 6; G. Kro: None; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; G. Grødeland: AstraZeneca, 1, Bayer, 6, Sanofi, 6, ThermoFisher, 6; L. Munthe: Celgene, 6, Novartis, 6; S. Mjaaland: None; J. Vaage: None; E. Haavardsholm: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Eli Lilly, 2, Gilead, 2, Pfizer, 6, UCB, 6; K. Jørgensen: Bristol-Myers Squibb(BMS), 6, Roche, 6; S. Provan: None; S. Syversen: AstraZeneca, 1; G. Goll: AbbVie/Abbott, 1, 6, Galapagos, 1, 6, Novartis, 6, Pfizer, 1, Union Chimique Belge, 1, 6.
Background/Purpose: Safety and efficacy of updated bivalent vaccines, containing both the original vaccine variant of SARS-CoV-2 Spike and either Omicron variants BA.1 or BA.4/5, are of particular interest in arthritis patients on immunosuppressive therapies. With the continuous emergence of new viral variants, it is important to evaluate whether updated vaccines induce more adverse events in this patient group. The objective of this study was examine if a second booster dose with updated bivalent vaccine increases the risk of adverse events, compared to the first booster dose with monovalent vaccines.
Methods: The prospective Nor-vaC study investigates vaccine responses in patients with immune mediated inflammatory diseases using immunosuppressive therapies (1). The present analyses included arthritis patients who received two booster doses. Patients received available vaccines according to the Norwegian vaccination program. The current recommendation in the Norwegian arthritis population is a three-dose primary vaccination series followed by two booster doses. Adverse events following vaccines doses were self-reported through questionnaires. Adverse events following the first (monovalent) and second (bivalent) booster were compared with McNemar’s test.
Results: Between 7th of July 2021 and 6th of December 2022 a total of 243 arthritis patients (127 rheumatoid arthritis, 65 psoriatic arthritis, 51 spondyloarthritis) on immunosuppressive therapies (Table) received a first, monovalent (BNT162b2, mRNA-1273) and a second, bivalent booster dose (BNT162b2 (WT/OMI BA.1), mRNA-1273.214, BNT162b2 (WT/OMI BA.4/BA.5)). Adverse events were recorded within two weeks in all patients (Figure). In total, 45 vs 49 (19% vs 20 %) patients reported any adverse event after a second, bivalent booster dose, compared to the first, monovalent booster, respectively. There was no significant difference in adverse events overall (p= 0.57). The most common adverse events after the second booster were pain at injection site (12 %), flu-like symptoms (9 %) and headache (6 %). No new safety signals emerged. A total of 15 (6 %) patients reported a disease flare after receiving the second, bivalent booster, compared to 21 (8 %) after the first, monovalent booster.
Conclusion: There was no difference in adverse events between the monovalent, first booster, and the bivalent, second booster, indicating that bivalent vaccines are safe in this patient group.
Reference:
1. Syversen S.W. et al Arthritis Rheumatol 2022

Figure: Adverse events after bivalent vaccine as a 2nd booster dose compared to a monovalent vaccine as a 1st booster dose.

Table: Demographic characteristics and immunosuppressive medication in patients receiving a 1st monovalent and a 2nd bivalent booster dose.
H. Ørbo: None; I. Jyssum: None; A. Tveter: None; I. Christensen: None; J. Sexton: None; K. Bjørlykke: Janssen-Cilag, 6; G. Kro: None; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; G. Grødeland: AstraZeneca, 1, Bayer, 6, Sanofi, 6, ThermoFisher, 6; L. Munthe: Celgene, 6, Novartis, 6; S. Mjaaland: None; J. Vaage: None; E. Haavardsholm: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Eli Lilly, 2, Gilead, 2, Pfizer, 6, UCB, 6; K. Jørgensen: Bristol-Myers Squibb(BMS), 6, Roche, 6; S. Provan: None; S. Syversen: AstraZeneca, 1; G. Goll: AbbVie/Abbott, 1, 6, Galapagos, 1, 6, Novartis, 6, Pfizer, 1, Union Chimique Belge, 1, 6.



