Poster Session A
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
0649: Early Systemic Sclerosis Definitions: Time to Rethink ‘Early’ in SSc Disease?
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- FD
Francesco Del Galdo, MD, PhD (he/him/his)
University of Leeds
Leeds, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Francesco Del Galdo1, Matthew Woods2, Mario Ehlers3 and Stefano Di Donato1, 1University of Leeds - Leeds Institute of Rheumatic and Muskuloskeletal Medicine, Leeds, United Kingdom, 2Lumanity, Manchester, United Kingdom, 3Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT
Background/Purpose: Systemic sclerosis (SSc) is a chronic autoimmune fibrotic disease with highly variable type and severity of organ involvement. Pathological changes in the skin, lungs, gastrointestinal tract, kidneys and heart determine clinical outcomes, with cardiopulmonary complications being the main cause of mortality, and musculokeletal changes and loss of hand function driving morbidity and disability. Early identification of the disease has been targeted in clinical trials in order to identify windows of opportunities for intervention, before organ damage becomes irreversible. Here we review how early SSc is defined in the literature and propose a paradigm shift in definition.
Methods: Structured, targeted searches were conducted by two reviewers in PubMed, Google Scholar, Google, and ClinicalTrials.gov in November 2021 and updated in March 2023. Search terms included 'early systemic sclerosis', 'early systemic sclerosis criteria', 'patients with early systemic sclerosis', 'opportunities in early systemic sclerosis', 'early scleroderma criteria' and 'early systemic sclerosis definition' to identify publications, treatment guidelines and clinical trial records.
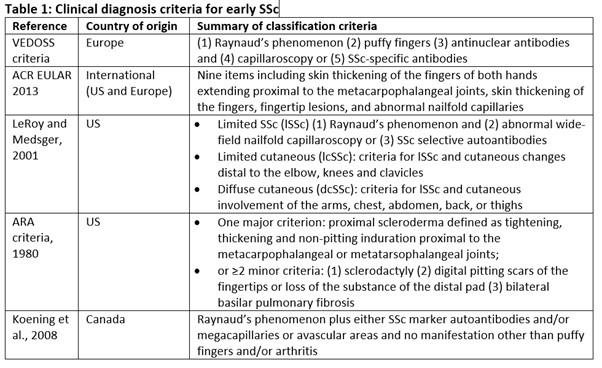
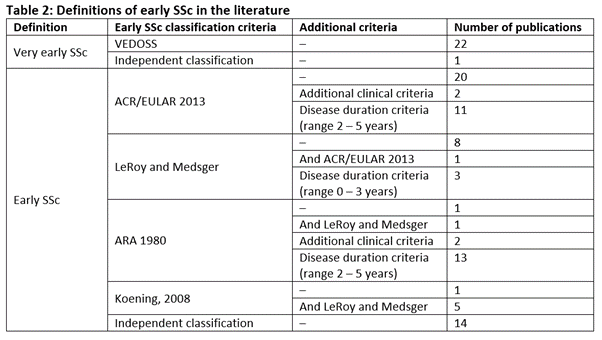
Results: Published evidence defining early SSc included 103 publications, three clinical guidelines and 16 clinical trial records. Early SSc is commonly defined by one of four classification criteria (ARA, 1980; LeRoy and Medsger; Koening, 2008; or ACR/EULAR, 2013) (Table 1), often alongside a time from first non-Raynaud's symptom (Table 2). However, there was no universal consensus on what this duration should be (generally ranging from 2 to 5 years). The key finding was the significant heterogeneity in definitions used across the literature (Table 2); however, there was consensus that Raynaud's phenomenon is usually the first sign. Some publications even used their own variations of the classification criteria. The aim of each of the main definitions was to improve the early identification of patients, with studies adding additional criteria to provide further clarity and/or to aid treatment decisions to avoid early disease damage and burden.
Conclusion: There is no clear consensus on the optimal clinical classification criteria or disease onset duration that should be used to define early SSc. This is likely due to the differing rationale behind existing research. A common drive for a definition of 'early', aligned with 'potentially reversible', may be true at different times across different organs. Since the goal to treat early SSc patients is to prevent irreversible organ damage, we would suggest that early is not considered as a factor of time, but of severity of organ involvement, and would recommend a consensus statement exercise to align on an appropriate definition.
F. Del Galdo: AbbVie/Abbott, 5, arxx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, capella, 2, Chemomab, 2, GlaxoSmithKlein(GSK), 2, Janssen, 2, Mitsubishi-Tanabe, 2, 5; M. Woods: None; M. Ehlers: Boehringer Ingelheim, 3; S. Di Donato: None.
Background/Purpose: Systemic sclerosis (SSc) is a chronic autoimmune fibrotic disease with highly variable type and severity of organ involvement. Pathological changes in the skin, lungs, gastrointestinal tract, kidneys and heart determine clinical outcomes, with cardiopulmonary complications being the main cause of mortality, and musculokeletal changes and loss of hand function driving morbidity and disability. Early identification of the disease has been targeted in clinical trials in order to identify windows of opportunities for intervention, before organ damage becomes irreversible. Here we review how early SSc is defined in the literature and propose a paradigm shift in definition.
Methods: Structured, targeted searches were conducted by two reviewers in PubMed, Google Scholar, Google, and ClinicalTrials.gov in November 2021 and updated in March 2023. Search terms included 'early systemic sclerosis', 'early systemic sclerosis criteria', 'patients with early systemic sclerosis', 'opportunities in early systemic sclerosis', 'early scleroderma criteria' and 'early systemic sclerosis definition' to identify publications, treatment guidelines and clinical trial records.
Results: Published evidence defining early SSc included 103 publications, three clinical guidelines and 16 clinical trial records. Early SSc is commonly defined by one of four classification criteria (ARA, 1980; LeRoy and Medsger; Koening, 2008; or ACR/EULAR, 2013) (Table 1), often alongside a time from first non-Raynaud's symptom (Table 2). However, there was no universal consensus on what this duration should be (generally ranging from 2 to 5 years). The key finding was the significant heterogeneity in definitions used across the literature (Table 2); however, there was consensus that Raynaud's phenomenon is usually the first sign. Some publications even used their own variations of the classification criteria. The aim of each of the main definitions was to improve the early identification of patients, with studies adding additional criteria to provide further clarity and/or to aid treatment decisions to avoid early disease damage and burden.
Conclusion: There is no clear consensus on the optimal clinical classification criteria or disease onset duration that should be used to define early SSc. This is likely due to the differing rationale behind existing research. A common drive for a definition of 'early', aligned with 'potentially reversible', may be true at different times across different organs. Since the goal to treat early SSc patients is to prevent irreversible organ damage, we would suggest that early is not considered as a factor of time, but of severity of organ involvement, and would recommend a consensus statement exercise to align on an appropriate definition.

Table 1: Clinical diagnosis criteria for early SSc

Table 2: Definitions of early SSc in the literature
F. Del Galdo: AbbVie/Abbott, 5, arxx, 2, AstraZeneca, 2, 5, Boehringer-Ingelheim, 2, 5, capella, 2, Chemomab, 2, GlaxoSmithKlein(GSK), 2, Janssen, 2, Mitsubishi-Tanabe, 2, 5; M. Woods: None; M. Ehlers: Boehringer Ingelheim, 3; S. Di Donato: None.



