Poster Session A
Rheumatoid arthritis (RA)
Session: (0380–0422) RA – Diagnosis, Manifestations, and Outcomes Poster I
0406: Obesity Is a Risk Factor for Poor Response to Treatment in Early Rheumatoid Arthritis – a NORD-STAR Spin-Off Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- Cm
Cristina maglio, MD, PhD
University of Gothenburg
Gothenburg, SwedenDisclosure information not submitted.
Abstract Poster Presenter(s)
Violetta Dubovyk1, Gerdur Maria Grondal2, Bjorn Gudbjornsson3, Espen A Haavardsholm4, Marte Schrumpf Heiberg4, Merete Hetland5, Kim Hørslev-Petersen6, Meliha Kapetanovic7, Alf Kastbom8, John Lampa9, Kristina Lend10, Dan Nordstrom11, Michael Nurmohamed12, Milad Rizk13, Annika Söderbergh14, Till Uhlig15, Mikkel Østergaard16, Ronald van Vollenhoven12, Anna Rudin17 and Cristina Maglio18, 1University of Gothenburg, Gothenburg, Sweden, 2Department for Rheumatology, Landspitali University Hospital, Reykjavik, Iceland, 3Centre for Rheumatology Research, University Hospital, Reykjavik, Iceland, 4Diakonhjemmet Hospital, Oslo, Norway, 5Copenhagen Center for Arthritis Research, Rigshospitalet, Copenhagen, Denmark, 6University of Southern Denmark, Odense, Denmark, 7Lund University and Skåne University Hospital, Lund, Sweden, 8Linköping University, Linköping, Sweden, 9Stockholm County, Hãsselby, Sweden, 10Amsterdam UMC, Karolinska Institute, Stockholm, Sweden, 11Helsinki University Hospital, Helsinki, Finland, 12Amsterdam University Medical Centers, Amsterdam, Netherlands, 13Västmanlands Hospital Västerås, Västerås, Sweden, 14Örebro University Hospital, Örebro, Sweden, 15Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, 16Copenhagen Center for Arthritis Research, Center for Rheumatology and Spine Diseases, Centre for Head and Orthopaedics, Rigshospitalet; University of Copenhagen, Copenhagen, Denmark, 17Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 18Institute of Medicine, University of Gothenburg, Gothenburg, Sweden
Background/Purpose: Several therapeutic options are currently available to treat rheumatoid arthritis (RA); however, the response to treatment is highly variable, and not all patients achieve clinical remission. Obesity is suggested to lower the chances of remission, even though a recent observational study has shown that obesity is not associated with reduced response to conventional synthetic anti-rheumatic drugs in patients with early RA. Here, we aim to determine if obesity affects the response to treatment in participants with early RA.
Methods: This report includes 393 Swedish patients from the NORD-STAR study, which is a multicenter, randomized trial on 812 patients with untreated early RA [4]. Participants have been randomized at baseline into 4 arms of treatment: methotrexate combined with (1) prednisolone, (2) certolizumab, (3) abatacept, or (4) tocilizumab. Scores for disease activity and blood samples were measured and collected before randomization and at 24-week follow-up.
Multiple linear regression and binary logistic regression analyses were performed adjusting for sex, baseline age, anti-citrullinated peptide antibody status, current smoking, disease activity score- C-reactive protein (DAS28-CRP), and treatment randomization. The outcomes for this report were: DAS28-CRP ≤3.2 (DAS28-CRP low disease activity), DAS28-CRP ≤2.6 (DAS28-CRP remission) and clinical disease activity index (CDAI) ≤2.8 (CDAI remission).
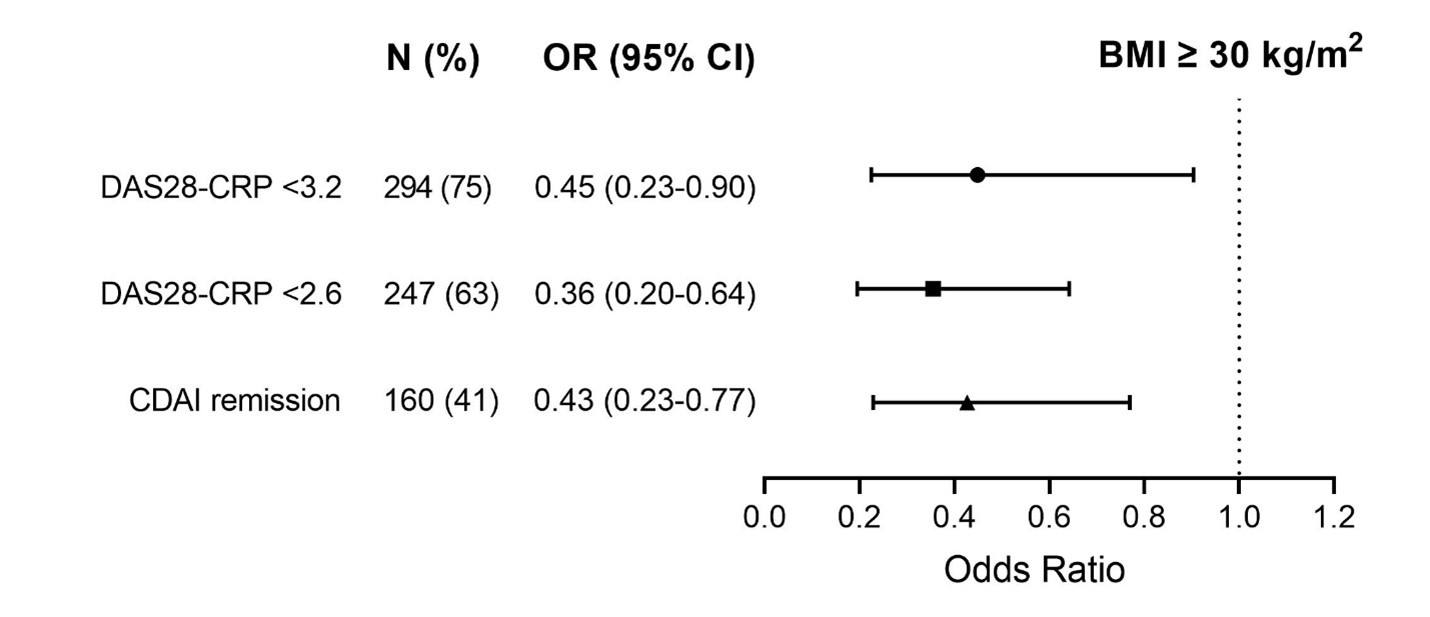
Results: In total, 75 (19%) participants had obesity at baseline, defined as body mass index (BMI) ≥30 kg/m2. The percentage of patients with obesity in each treatment group was (1) 25%, (2) 15%, (3) 19% and (4) 19%. At baseline, there were no differences in terms of disease activity indices and inflammation parameters between patients with BMI <30 vs. ≥30 kg/m2, except for number of swollen joints (SJC28), which was slightly lower in those with obesity (mean+SD, 8±5 vs. 9±5, p=0.018). At 24-week follow-up, patients with obesity had higher disease activity indices and inflammation parameters compared to those with lower BMI (Table 1). Moreover, patients with obesity had a lower chance of achieving response to treatment as measured by DAS28-CRP ≤3.2 (OR 0.5, 95% CI 0.2 - 0.9, p=0.025), DAS28-CRP ≤2.6 (OR 0.4, 95% CI 0.2 - 0.6, p < 0.001) and CDAI remission (OR 0.4, 95% CI 0.2 - 0.8, p=0.006), compared to patients with lower BMI (Fig. 1). BMI-treatment interaction was not significant for any score of disease activity.
Conclusion: In patients with early RA, obesity was not associated with higher disease activity before treatment initiation. However, 24 weeks after treatment, patients with obesity had higher disease activity and lower chances to respond to therapy compared to patients with lower BMI irrespective of treatment.
.jpg)
V. Dubovyk: None; G. Grondal: None; B. Gudbjornsson: Nordic-Pharma, 6, Novartis, 2, 6; E. Haavardsholm: None; M. Schrumpf Heiberg: Roche, 6; M. Hetland: AbbVie/Abbott, 1, 5, Bristol-Myers Squibb(BMS), 5, Danbio, 12, Chari of Danbio registry, Eli Lilly, 5, MEDAC, 6, Novartis, 5, Pfizer, 5, 6, Sandoz, 5, 6; K. Hørslev-Petersen: None; M. Kapetanovic: None; A. Kastbom: None; J. Lampa: None; K. Lend: None; D. Nordstrom: AbbVie/Abbott, 2, BMS, 2, Lilly, 2, MSD, 2, Novartis, 2, Pfizer, 2, UCB, 2; M. Nurmohamed: None; M. Rizk: None; A. Söderbergh: None; T. Uhlig: Galapagos, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB, 2, 6; M. Østergaard: AbbVie, 2, 5, 6, Amgen, 5, Boehringer-Ingelheim, 2, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Celgene, 2, 5, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, Hospira, 2, 6, Janssen, 2, 6, MEDAC, 6, Merck, 2, 5, 6, Novartis, 2, 5, 6, Novo Nordisk, 2, 6, Orion, 2, 6, Pfizer, 2, 6, Regeneron, 2, 6, Roche, 2, 6, Sandoz, 2, 6, Sanofi, 2, 6, UCB, 2, 6; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 5, 6, Biogen, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 5, 6, GlaxoSmithKline, 6, Janssen, 2, 6, MSD/Merck Sharp and Dohme, 5, Novartis, 5, Pfizer, 2, 5, 6, RemeGen, 2, Roche, 5, Sanofi, 5, UCB, 2, 5, 6; A. Rudin: AstraZeneca, 12, financial support; C. Maglio: None.
Background/Purpose: Several therapeutic options are currently available to treat rheumatoid arthritis (RA); however, the response to treatment is highly variable, and not all patients achieve clinical remission. Obesity is suggested to lower the chances of remission, even though a recent observational study has shown that obesity is not associated with reduced response to conventional synthetic anti-rheumatic drugs in patients with early RA. Here, we aim to determine if obesity affects the response to treatment in participants with early RA.
Methods: This report includes 393 Swedish patients from the NORD-STAR study, which is a multicenter, randomized trial on 812 patients with untreated early RA [4]. Participants have been randomized at baseline into 4 arms of treatment: methotrexate combined with (1) prednisolone, (2) certolizumab, (3) abatacept, or (4) tocilizumab. Scores for disease activity and blood samples were measured and collected before randomization and at 24-week follow-up.
Multiple linear regression and binary logistic regression analyses were performed adjusting for sex, baseline age, anti-citrullinated peptide antibody status, current smoking, disease activity score- C-reactive protein (DAS28-CRP), and treatment randomization. The outcomes for this report were: DAS28-CRP ≤3.2 (DAS28-CRP low disease activity), DAS28-CRP ≤2.6 (DAS28-CRP remission) and clinical disease activity index (CDAI) ≤2.8 (CDAI remission).
Results: In total, 75 (19%) participants had obesity at baseline, defined as body mass index (BMI) ≥30 kg/m2. The percentage of patients with obesity in each treatment group was (1) 25%, (2) 15%, (3) 19% and (4) 19%. At baseline, there were no differences in terms of disease activity indices and inflammation parameters between patients with BMI <30 vs. ≥30 kg/m2, except for number of swollen joints (SJC28), which was slightly lower in those with obesity (mean+SD, 8±5 vs. 9±5, p=0.018). At 24-week follow-up, patients with obesity had higher disease activity indices and inflammation parameters compared to those with lower BMI (Table 1). Moreover, patients with obesity had a lower chance of achieving response to treatment as measured by DAS28-CRP ≤3.2 (OR 0.5, 95% CI 0.2 - 0.9, p=0.025), DAS28-CRP ≤2.6 (OR 0.4, 95% CI 0.2 - 0.6, p < 0.001) and CDAI remission (OR 0.4, 95% CI 0.2 - 0.8, p=0.006), compared to patients with lower BMI (Fig. 1). BMI-treatment interaction was not significant for any score of disease activity.
Conclusion: In patients with early RA, obesity was not associated with higher disease activity before treatment initiation. However, 24 weeks after treatment, patients with obesity had higher disease activity and lower chances to respond to therapy compared to patients with lower BMI irrespective of treatment.
.jpg)
Table 1. Disease activity scores and inflammatory parameters at 24 weeks follow-up stratified by baseline BMI.
Abbreviations: TJC28, tender joint count; VAS, visual analogue scale of pain; ESR, erythrocyte sedimentation rate.
Abbreviations: TJC28, tender joint count; VAS, visual analogue scale of pain; ESR, erythrocyte sedimentation rate.

Fig. 1. Multivariable binary logistic regression analysis for response to treatment at 24 weeks according to BMI (reference category: BMI< 30 kg/m2).
V. Dubovyk: None; G. Grondal: None; B. Gudbjornsson: Nordic-Pharma, 6, Novartis, 2, 6; E. Haavardsholm: None; M. Schrumpf Heiberg: Roche, 6; M. Hetland: AbbVie/Abbott, 1, 5, Bristol-Myers Squibb(BMS), 5, Danbio, 12, Chari of Danbio registry, Eli Lilly, 5, MEDAC, 6, Novartis, 5, Pfizer, 5, 6, Sandoz, 5, 6; K. Hørslev-Petersen: None; M. Kapetanovic: None; A. Kastbom: None; J. Lampa: None; K. Lend: None; D. Nordstrom: AbbVie/Abbott, 2, BMS, 2, Lilly, 2, MSD, 2, Novartis, 2, Pfizer, 2, UCB, 2; M. Nurmohamed: None; M. Rizk: None; A. Söderbergh: None; T. Uhlig: Galapagos, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB, 2, 6; M. Østergaard: AbbVie, 2, 5, 6, Amgen, 5, Boehringer-Ingelheim, 2, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Celgene, 2, 5, 6, Eli Lilly, 2, 6, Galapagos, 2, 6, Gilead, 2, 6, Hospira, 2, 6, Janssen, 2, 6, MEDAC, 6, Merck, 2, 5, 6, Novartis, 2, 5, 6, Novo Nordisk, 2, 6, Orion, 2, 6, Pfizer, 2, 6, Regeneron, 2, 6, Roche, 2, 6, Sandoz, 2, 6, Sanofi, 2, 6, UCB, 2, 6; R. van Vollenhoven: AbbVie, 2, 6, AstraZeneca, 2, 5, 6, Biogen, 6, Bristol-Myers Squibb(BMS), 2, 5, 6, Galapagos, 2, 5, 6, GlaxoSmithKline, 6, Janssen, 2, 6, MSD/Merck Sharp and Dohme, 5, Novartis, 5, Pfizer, 2, 5, 6, RemeGen, 2, Roche, 5, Sanofi, 5, UCB, 2, 5, 6; A. Rudin: AstraZeneca, 12, financial support; C. Maglio: None.



