Poster Session A
Crystal arthropathies
Session: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
0244: Efficacy and Safety of AR882, a Selective Uric Acid Transporter 1 (URAT1) Inhibitor, in Gout Patients with Various Baseline Characteristics Following 12-Week Treatment in Patients
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- RK
Robert T. Keenan, MD, MPH, MBA
Arthrosi Therapeutics
Chapel Hill, NC, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
James Cheng-Chung Wei1, Roy Fleischmann2, sarah Morris3, Vijay Hingorani4, Elizabeth Polvent5, Zancong Shen6, Shunqi Yan7, Li-Tain Yeh8 and Robert Keenan9, 1Chung Shan Medical University Hospital, Department of Rheumatology, Taichung, Taiwan, 2Division of Rheumatology, University of Texas Southwestern Medical Center, Metroplex Clinical Research Center, Dallas, TX, 3Arthrosi Therapeutics Inc, San Diego, CA, 4Vanguard Healthsciences, Inc., San Diego, CA, 5Arthrosi Therapeutics, Inc., Roseville, CA, 6Arthrosi Therapeutics, San Diego, CA, 7Arthrosi Therapeutics, Inc., Laguna Hills, CA, 8Arthrosi Therapeutics, Inc., Irvine, CA, 9Arthrosi Therapeutics, Chapel Hill, NC
Background/Purpose: AR882 is a novel, potent, and selective URAT1inhibitor in development for the treatment of gout and tophaceous gout. AR882-202 was a global, multi-center, randomized, double-blinded, 12-week, placebo-controlled phase 2b trial to evaluate the safety and efficacy of AR882 versus placebo in patients with gout. Here, we report the analysis of the efficacy and safety based on various demographics and baseline characteristics.
Methods: The trial recruited 140 gout patients 18 to 75 years of age with eGFR >30 mL/min across 20 sites in the US, Australia, and Taiwan. Subjects were recruited from the US (15 sites), Australia (2 sites) and Taiwan (3 sites), and were randomized into one of three treatment groups at a 1:1:1 ratio. Blood samples were collected every 2 weeks up to 12 weeks to monitor sUA levels and safety measures. Efficacy endpoints included the percent of patients who reached a sUA < 6, < 5 mg/dL and lower. Safety data, including vital signs and electrocardiograms, were collected throughout the study.
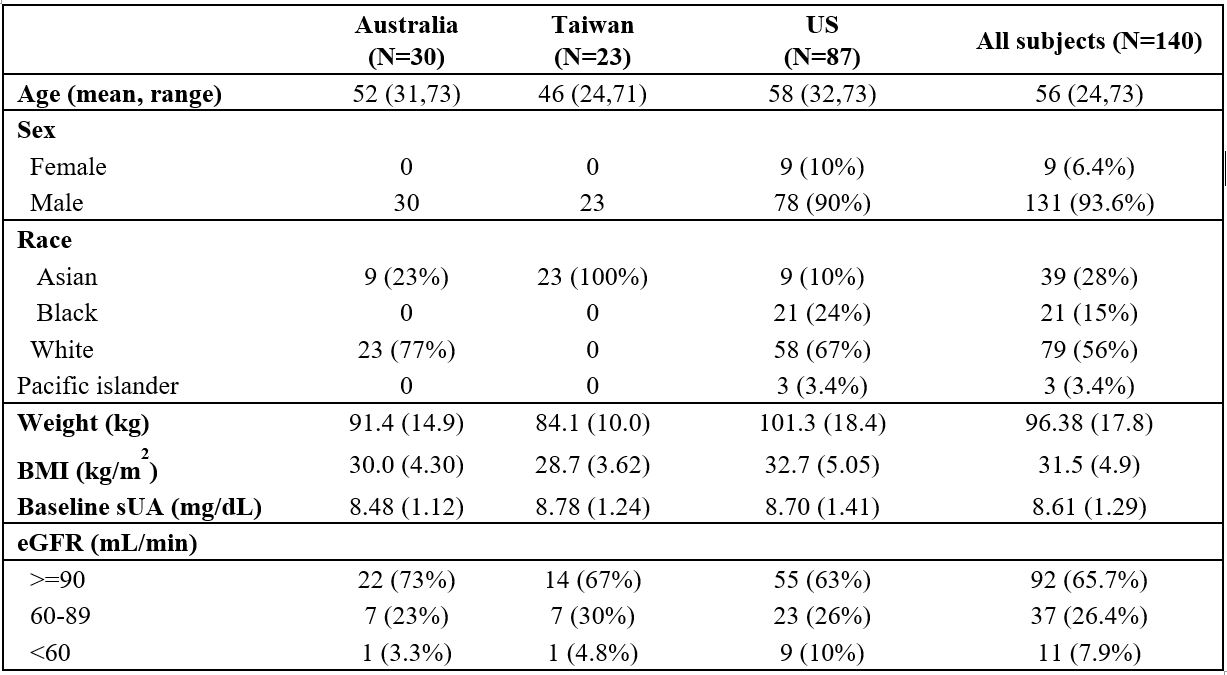
Results: Baseline characteristics of enrolled participants are illustrated in Table 1. The mean baseline sUA level was 8.6 (±1.3) mg/dL and was similar across regions (8.5-8.8 mg/dL), while mean body weights and BMI showed slight regional differences.
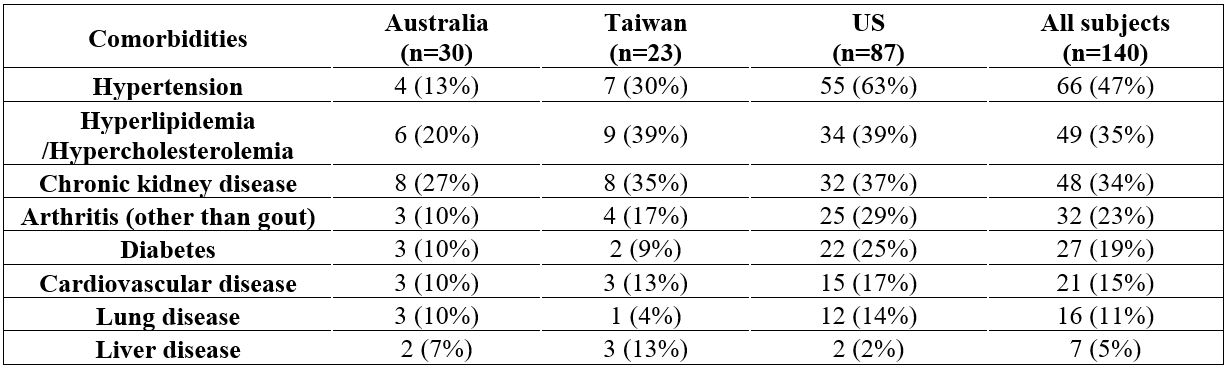
Common comorbidities in participants included hypertension, hyperlipidemia, chronic kidney disease, followed by arthritis, diabetes, and cardiovascular disease. The US population had a higher rate of comorbidities than those in Australia and Taiwan (Table 2).
In the intent-to-treat population, in the 75 mg group 82% and 73% of patients achieved sUA < 6 and < 5 mg/dL, respectively, and in the 50 mg group, 71% and 45% of patients achieved sUA levels < 6 and < 5 mg/dL, respectively. In the per-protocol population, in the 75 mg group 89% and 82% achieved sUA levels < 6 and < 5 mg/dL, respectively, and in the 50 mg group 78% and 50% achieved sUA levels < 6 and < 5 mg/dL, respectively. Patients in Australia and Taiwan showed slightly higher response rates than those in the US (Table 3), likely attributable to the difference in body weight and BMI. Patients with eGFR in the 60-89 mL/min range showed similar response rates to those with eGFR > 90 mL/min, and greater response rates than those with eGFR < 60 mL/min. There were no serious adverse events in AR882 treated patients. The most frequently reported adverse event was gout flare occurring in 30% of patients overall with similar distribution among placebo and AR882 treatment groups. Mild or moderate adverse events including diarrhea, headache, and upper respiratory infection were observed. None of the AEs led to discontinuation of investigational product.
Conclusion: 12-week treatment of AR882 demonstrated safe and efficacious profiles in gout patients with various demographics and baseline characteristics. AR882 may offer improved efficacy and better safety compared to existing therapies in the treatment of patients with gout including those with severe or refractory disease across various demographics and comorbidities.
.jpg)
J. Wei: Abbvie, 2, 5, 6, Amgen, 5, AstraZeneca, 6, BMS, 2, 5, 6, Celgene, 2, Chugai, 2, 6, Eisai, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 5, GSK, 2, 5, Janssen, 2, 5, 6, Novartis, 2, 5, Pfizer, 2, 5, 6, Sanofi-Aventis, 2, SUN pharma, 5, TSH Taiwan, 2, UCB pharma, 2, 5; R. Fleischmann: AbbVie, 1, 2, 5, Amgen, 1, 2, 5, Bristol Myers Squibb, 1, 2, 5, Eli Lilly, 1, 2, 5, Galapagos, 1, 2, 5, Galvani, 1, 2, 5, Gilead, 1, 2, 5, GlaxoSmithKline, 1, 2, 5, Janssen, 1, 2, 5, Novartis, 1, 2, 5, Pfizer, 1, 2, 5, UCB, 1, 2, 5, Vyne, 1, 2, 5; s. Morris: Arthrosi Therapeutics, 3; V. Hingorani: None; E. Polvent: Arthrosi Therapeutics, 3; Z. Shen: Arthrosi therapeutics, 3; S. Yan: Arthrosi Therapeutics, 3; L. Yeh: Arthrosi Therapeutics, 3; R. Keenan: Arthrosi Therapeutics, 3.
Background/Purpose: AR882 is a novel, potent, and selective URAT1inhibitor in development for the treatment of gout and tophaceous gout. AR882-202 was a global, multi-center, randomized, double-blinded, 12-week, placebo-controlled phase 2b trial to evaluate the safety and efficacy of AR882 versus placebo in patients with gout. Here, we report the analysis of the efficacy and safety based on various demographics and baseline characteristics.
Methods: The trial recruited 140 gout patients 18 to 75 years of age with eGFR >30 mL/min across 20 sites in the US, Australia, and Taiwan. Subjects were recruited from the US (15 sites), Australia (2 sites) and Taiwan (3 sites), and were randomized into one of three treatment groups at a 1:1:1 ratio. Blood samples were collected every 2 weeks up to 12 weeks to monitor sUA levels and safety measures. Efficacy endpoints included the percent of patients who reached a sUA < 6, < 5 mg/dL and lower. Safety data, including vital signs and electrocardiograms, were collected throughout the study.
Results: Baseline characteristics of enrolled participants are illustrated in Table 1. The mean baseline sUA level was 8.6 (±1.3) mg/dL and was similar across regions (8.5-8.8 mg/dL), while mean body weights and BMI showed slight regional differences.
Common comorbidities in participants included hypertension, hyperlipidemia, chronic kidney disease, followed by arthritis, diabetes, and cardiovascular disease. The US population had a higher rate of comorbidities than those in Australia and Taiwan (Table 2).
In the intent-to-treat population, in the 75 mg group 82% and 73% of patients achieved sUA < 6 and < 5 mg/dL, respectively, and in the 50 mg group, 71% and 45% of patients achieved sUA levels < 6 and < 5 mg/dL, respectively. In the per-protocol population, in the 75 mg group 89% and 82% achieved sUA levels < 6 and < 5 mg/dL, respectively, and in the 50 mg group 78% and 50% achieved sUA levels < 6 and < 5 mg/dL, respectively. Patients in Australia and Taiwan showed slightly higher response rates than those in the US (Table 3), likely attributable to the difference in body weight and BMI. Patients with eGFR in the 60-89 mL/min range showed similar response rates to those with eGFR > 90 mL/min, and greater response rates than those with eGFR < 60 mL/min. There were no serious adverse events in AR882 treated patients. The most frequently reported adverse event was gout flare occurring in 30% of patients overall with similar distribution among placebo and AR882 treatment groups. Mild or moderate adverse events including diarrhea, headache, and upper respiratory infection were observed. None of the AEs led to discontinuation of investigational product.
Conclusion: 12-week treatment of AR882 demonstrated safe and efficacious profiles in gout patients with various demographics and baseline characteristics. AR882 may offer improved efficacy and better safety compared to existing therapies in the treatment of patients with gout including those with severe or refractory disease across various demographics and comorbidities.

Table 1.Demographics and baseline characteristics

Table 2. Patient comorbidities across regions
.jpg)
Table 3. Response rate of sUA lowering in patients across regions (per-protocol population)
J. Wei: Abbvie, 2, 5, 6, Amgen, 5, AstraZeneca, 6, BMS, 2, 5, 6, Celgene, 2, Chugai, 2, 6, Eisai, 2, 6, Eli Lilly, 2, 5, 6, Gilead, 5, GSK, 2, 5, Janssen, 2, 5, 6, Novartis, 2, 5, Pfizer, 2, 5, 6, Sanofi-Aventis, 2, SUN pharma, 5, TSH Taiwan, 2, UCB pharma, 2, 5; R. Fleischmann: AbbVie, 1, 2, 5, Amgen, 1, 2, 5, Bristol Myers Squibb, 1, 2, 5, Eli Lilly, 1, 2, 5, Galapagos, 1, 2, 5, Galvani, 1, 2, 5, Gilead, 1, 2, 5, GlaxoSmithKline, 1, 2, 5, Janssen, 1, 2, 5, Novartis, 1, 2, 5, Pfizer, 1, 2, 5, UCB, 1, 2, 5, Vyne, 1, 2, 5; s. Morris: Arthrosi Therapeutics, 3; V. Hingorani: None; E. Polvent: Arthrosi Therapeutics, 3; Z. Shen: Arthrosi therapeutics, 3; S. Yan: Arthrosi Therapeutics, 3; L. Yeh: Arthrosi Therapeutics, 3; R. Keenan: Arthrosi Therapeutics, 3.



