Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0582–0608) SLE – Treatment Poster I
0595: Renal Involvement in Patients with Systemic Lupus Erythematosus Treated with Anifrolumab Compared with Placebo over a 4-Year Period
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall

Richard Furie, MD
Chief of the Division of Rheumatology
Northwell Health
Manhasset, NY, NY, United StatesDisclosure(s): Biogen: Consultant (Ongoing), Grant/Research Support (Ongoing)
Abstract Poster Presenter(s)
Richard A. Furie1, Kenneth Kalunian2, Eric Morand3, Ian Bruce4, Susan Manzi5, Gelareh Atefi6, Gary Bryant6, Micki Hultquist7, Raj Tummala7, Gabriel Abreu8, Catharina Lindholm8 and Hussein Al-Mossawi9, 1Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Great Neck, NY, 2University of California San Diego, La Jolla, CA, 3Monash University, Centre for Inflammatory Diseases, Melbourne, Australia, 4University of Manchester, Manchester, United Kingdom, 5Lupus Center of Excellence, Autoimmunity Institute, Allegheny Health Network, Pittsburgh, PA, 6BioPharmaceuticals Medical, AstraZeneca, Wilmington, DE, 7BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, MD, 8BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden, 9BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom
Background/Purpose: In patients with SLE, nephritis is present in 50%–60% during the first 10 years of disease.1 Renal involvement is associated with poor clinical outcomes including increased morbidity and mortality.1,2 Anifrolumab is a human mAb to the type I IFN receptor that is approved for the treatment of SLE.3 In this post hoc analysis of the phase 3 TULIP long-term extension (LTE) trial of anifrolumab, we assessed renal outcomes in patients with SLE with or without evidence of SLEDAI-2K renal involvement at baseline.4
Methods: Adults with moderate to severe SLE (1997 ACR criteria) despite standard therapy who completed the 52-week, multicenter phase 3 TULIP-1 (NCT02446912) or TULIP-2 (NCT02446899) trials could reconsent to participate in the randomized, placebo-controlled, double-blind, 3-year extension (NCT02794285). The TULIP-1 and TULIP-2 trials excluded patients with active, severe lupus nephritis, serum creatinine >2 mg/dL, or urine protein/creatinine ratio >2 mg/mg. We identified patients with renal involvement below the threshold for exclusion at baseline, or patients without renal involvement, who were randomized to receive intravenous anifrolumab 300 mg or placebo across the TULIP-1/-2 and LTE periods (or would have continued the same treatment in the LTE if not discontinued during TULIP-1/-2). SLEDAI-2K renal domain involvement was assessed from baseline through Week 208.
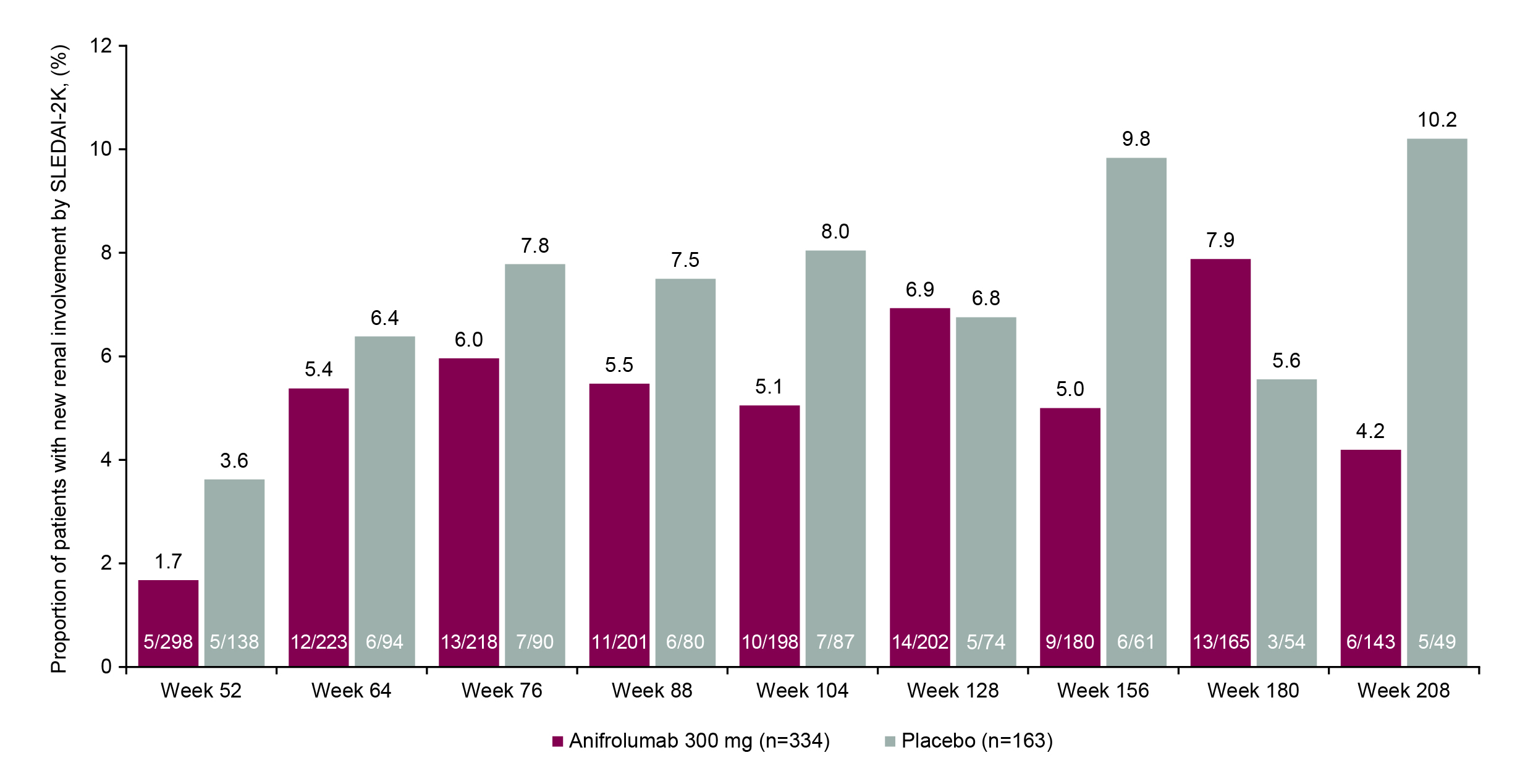
Results: At TULIP baseline, the proportions of patients with SLEDAI-2K renal involvement were balanced between treatment groups (anifrolumab 6.7% [24/358] vs placebo 8.4% [15/178]). Of patients with baseline renal involvement and available data, a greater proportion of patients achieved renal improvement at LTE study entry (Week 52) with anifrolumab vs placebo (70.6% [12/17] vs 50.0% [6/12]). A greater proportion of patients with baseline renal involvement continued treatment to Week 208 with anifrolumab vs placebo (33.3% [8/24] vs 6.7% [1/15]), and renal improvement at Week 208 was achieved by more patients who received anifrolumab (90.0% [9/10]) vs placebo (0% [0 patients with available data at this timepoint]). Of all patients included in this analysis, ≤1/358 in the anifrolumab group and ≤3/178 in the placebo group experienced new renal activity greater than the baseline score at any timepoint during the study period. In patients with no baseline renal involvement, lower proportions of patients had new renal activity with anifrolumab vs placebo at all timepoints up to Week 208, except for Weeks 128 and 180 (Figure).
Conclusion: In the small subgroup of patients with renal involvement at baseline, more
patients treated with anifrolumab remained in the study and achieved SLEDAI-2K renal improvement compared with placebo over the 4-year TULIP-LTE trial. Of patients with no renal involvement at baseline, fewer patients had new renal activity with anifrolumab compared with placebo.
References:
R. Furie: AstraZeneca, 2, 5, 6; K. Kalunian: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Biogen, 2, Bristol-Myers Squibb, 2, Eli Lilly, 2, Genentech/Roche, 2, GlaxoSmithKline, 2, Janssen, 2, Pfizer, 2; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; I. Bruce: AstraZeneca, 1, 2, 5, 6, Aurinia, 2, GSK, 1, 2, 5, 6, Janssen, 5, 6, Lilly, 1, UCB, 6; S. Manzi: AbbVie, 5, Allegheny Singer Research Institute, 10, AstraZeneca, 2, 5, Exagen Diagnostics, Inc, 2, 9, 10, GSK, 2, 5, Lilly, 2, Lupus Foundation of America, 4, Novartis, 2, UCB Advisory Board, 2, Univiersity of Pittsburgh, 10; G. Atefi: AstraZeneca, 3; G. Bryant: AstraZeneca, 3; M. Hultquist: AstraZeneca, 3, 11; R. Tummala: AstraZeneca, 3; G. Abreu: AstraZeneca, 3; C. Lindholm: AstraZeneca, 3; H. Al-Mossawi: AstraZeneca, 3.
Background/Purpose: In patients with SLE, nephritis is present in 50%–60% during the first 10 years of disease.1 Renal involvement is associated with poor clinical outcomes including increased morbidity and mortality.1,2 Anifrolumab is a human mAb to the type I IFN receptor that is approved for the treatment of SLE.3 In this post hoc analysis of the phase 3 TULIP long-term extension (LTE) trial of anifrolumab, we assessed renal outcomes in patients with SLE with or without evidence of SLEDAI-2K renal involvement at baseline.4
Methods: Adults with moderate to severe SLE (1997 ACR criteria) despite standard therapy who completed the 52-week, multicenter phase 3 TULIP-1 (NCT02446912) or TULIP-2 (NCT02446899) trials could reconsent to participate in the randomized, placebo-controlled, double-blind, 3-year extension (NCT02794285). The TULIP-1 and TULIP-2 trials excluded patients with active, severe lupus nephritis, serum creatinine >2 mg/dL, or urine protein/creatinine ratio >2 mg/mg. We identified patients with renal involvement below the threshold for exclusion at baseline, or patients without renal involvement, who were randomized to receive intravenous anifrolumab 300 mg or placebo across the TULIP-1/-2 and LTE periods (or would have continued the same treatment in the LTE if not discontinued during TULIP-1/-2). SLEDAI-2K renal domain involvement was assessed from baseline through Week 208.
Results: At TULIP baseline, the proportions of patients with SLEDAI-2K renal involvement were balanced between treatment groups (anifrolumab 6.7% [24/358] vs placebo 8.4% [15/178]). Of patients with baseline renal involvement and available data, a greater proportion of patients achieved renal improvement at LTE study entry (Week 52) with anifrolumab vs placebo (70.6% [12/17] vs 50.0% [6/12]). A greater proportion of patients with baseline renal involvement continued treatment to Week 208 with anifrolumab vs placebo (33.3% [8/24] vs 6.7% [1/15]), and renal improvement at Week 208 was achieved by more patients who received anifrolumab (90.0% [9/10]) vs placebo (0% [0 patients with available data at this timepoint]). Of all patients included in this analysis, ≤1/358 in the anifrolumab group and ≤3/178 in the placebo group experienced new renal activity greater than the baseline score at any timepoint during the study period. In patients with no baseline renal involvement, lower proportions of patients had new renal activity with anifrolumab vs placebo at all timepoints up to Week 208, except for Weeks 128 and 180 (Figure).
Conclusion: In the small subgroup of patients with renal involvement at baseline, more
patients treated with anifrolumab remained in the study and achieved SLEDAI-2K renal improvement compared with placebo over the 4-year TULIP-LTE trial. Of patients with no renal involvement at baseline, fewer patients had new renal activity with anifrolumab compared with placebo.
References:
- Hahn BH, et al. Arthritis Care Res (Hoboken). 2012;64:797–808.
- Hanly JG, et al. Rheumatology (Oxford). 2016;55:252–62.
- SAPHNELO Prescribing Information, Wilmington, DE: AstraZeneca.
- Kalunian KC, et al. Arthritis Rheumatol. 2023;75:253–65.

Figure. New Renal Involvement During the LTE Period Among Patients With No Renal Involvement at TULIP Baseline.
No renal involvement was defined as renal SLEDAI-2K score =0.
New renal involvement was defined as renal SLEDAI-2K score >0.
For measuring no renal involvement, percentages are based upon all patients in the full analysis set.
For measuring new renal involvement, percentages are based upon all patients in the full analysis set with no involvement at baseline and according to the number assessed at each timepoint thereafter; patients are missing due to either discontinuation, withdrawal, or missing data.
Baseline means baseline of the phase 3 feeder studies, ie, the last non-missing measurement prior to dose administration on Day 1.
No renal involvement was defined as renal SLEDAI-2K score =0.
New renal involvement was defined as renal SLEDAI-2K score >0.
For measuring no renal involvement, percentages are based upon all patients in the full analysis set.
For measuring new renal involvement, percentages are based upon all patients in the full analysis set with no involvement at baseline and according to the number assessed at each timepoint thereafter; patients are missing due to either discontinuation, withdrawal, or missing data.
Baseline means baseline of the phase 3 feeder studies, ie, the last non-missing measurement prior to dose administration on Day 1.
R. Furie: AstraZeneca, 2, 5, 6; K. Kalunian: AbbVie, 2, Amgen, 2, AstraZeneca, 2, Biogen, 2, Bristol-Myers Squibb, 2, Eli Lilly, 2, Genentech/Roche, 2, GlaxoSmithKline, 2, Janssen, 2, Pfizer, 2; E. Morand: AbbVie, 2, 5, Amgen, 5, AstraZeneca, 2, 5, 6, Biogen, 2, 5, Bristol Myers Squibb, 2, 5, Eli Lilly, 2, 5, EMD Serono, 2, 5, Galapagos, 2, Genentech, 2, 5, GlaxoSmithKline, 2, 5, IgM, 2, Janssen, 2, 5, Novartis, 2, Servier, 2, Takeda, 2, UCB, 5; I. Bruce: AstraZeneca, 1, 2, 5, 6, Aurinia, 2, GSK, 1, 2, 5, 6, Janssen, 5, 6, Lilly, 1, UCB, 6; S. Manzi: AbbVie, 5, Allegheny Singer Research Institute, 10, AstraZeneca, 2, 5, Exagen Diagnostics, Inc, 2, 9, 10, GSK, 2, 5, Lilly, 2, Lupus Foundation of America, 4, Novartis, 2, UCB Advisory Board, 2, Univiersity of Pittsburgh, 10; G. Atefi: AstraZeneca, 3; G. Bryant: AstraZeneca, 3; M. Hultquist: AstraZeneca, 3, 11; R. Tummala: AstraZeneca, 3; G. Abreu: AstraZeneca, 3; C. Lindholm: AstraZeneca, 3; H. Al-Mossawi: AstraZeneca, 3.



