Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Treatments III: Predictors of Response & Tapering (2539–2544)
2543: Clinical and Radiographic Results of Tapering and Withdrawing CsDMARDs versus Stable Treatment in Patients with Rheumatoid Arthritis in Remission: 3-year Results from a Randomized Controlled Trial
Tuesday, November 14, 2023
5:00 PM - 5:10 PM PT
Location: Ballroom 20B-C

Siri Lillegraven, MD, PhD, MPH
Diakonhjemmet Hospital
Oslo, NorwayDisclosure(s): Boehringer-Ingelheim: Grant/Research Support (Ongoing)
Presenting Author(s)
Kaja Eriksrud Kjørholt1, Nina Paulshus Sundlisæter1, Anna-Birgitte Aga1, Joseph Sexton1, Inge Olsen2, Hallvard Fremstad3, Cristina Spada4, Tor Magne Madland5, Christian A Høili6, Gunnstein Bakland7, Åse Lexberg8, Inger Johanne Widding Hansen9, Inger Myrnes Hansen10, Hilde Haukland11, Maud-Kristine Aga Ljoså12, Ellen Moholt1, Till Uhlig1, Tore Kvien1, Daniel Solomon13, Désirée van der Heijde14, Espen Haavardsholm1 and Siri Lillegraven1, 1Center for Treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, 2Oslo University Hospital, Oslo, Norway, 3Møre og Romsdal Hospital Trust, Ålesund, Norway, 4Lillehammer Hospital for Rheumatic Diseases, Lillehammer, Norway, 5Haukeland University Hospital, Bergen, Norway, 6Østfold Hospital Trust, Moss, Norway, 7University Hospital of North Norway, Tromsø, Norway, 8Drammen Hospital, Vestre Viken HF, Drammen, Norway, 9Sørlandet Hospital HF, Kristiansand, Norway, 10Helgeland Hospital Trust Mo i Rana, Mo i Rana, Norway, 11Martina Hansens Hospital, Bærum, Norway, 12Ålesund Hospital, Ålesund, Norway, 13Brigham and Women's Hospital, Newton, MA, 14Department of Rheumatology, Leiden University Medical Center, Leiden, Netherlands
Background/Purpose: Tapering of disease-modifying antirheumatic drugs (DMARDs) to achieve drug-free remission is a potential goal for the growing group of patients with rheumatoid arthritis (RA) in remission, although long-term effects of such strategies remain unclear. The purpose of this study was to compare the 3-year clinical and radiographic outcomes of three conventional synthetic DMARD (csDMARD) treatment strategies (continued stable treatment, half-dose treatment and tapering to withdrawal) among patients in sustained RA remission.
Methods: ARCTIC REWIND was a randomized, multicenter, open-label, clinical trial enrolling 160 RA patients in sustained remission for ≥1 year on stable csDMARD therapy (Lillegraven et al. JAMA 2021). Patients were randomized 2:1:1 to stable csDMARDs, half-dose csDMARDs, or half-dose csDMARDs for one year followed by withdrawal of all csDMARDs. The primary endpoint was absence of disease activity flare over 3 years. A flare was defined as a combination of DAS >1.6, an increase in DAS ≥0.6 units and ≥2 swollen joints, or if the physician and patient agreed that a clinically significant flare had occurred. Full-dose csDMARD treatment was reinstated upon flare. Secondary endpoints included remission (DAS, ACR/EULAR Boolean), and the 3-year change in radiographic joint damage (van der Heijde-Sharp score, vdHSS). Data were analyzed using Kaplan-Meier, Mann-Whitney test, Cox and mixed effect logistic regression, with stratification or adjustment for study center.
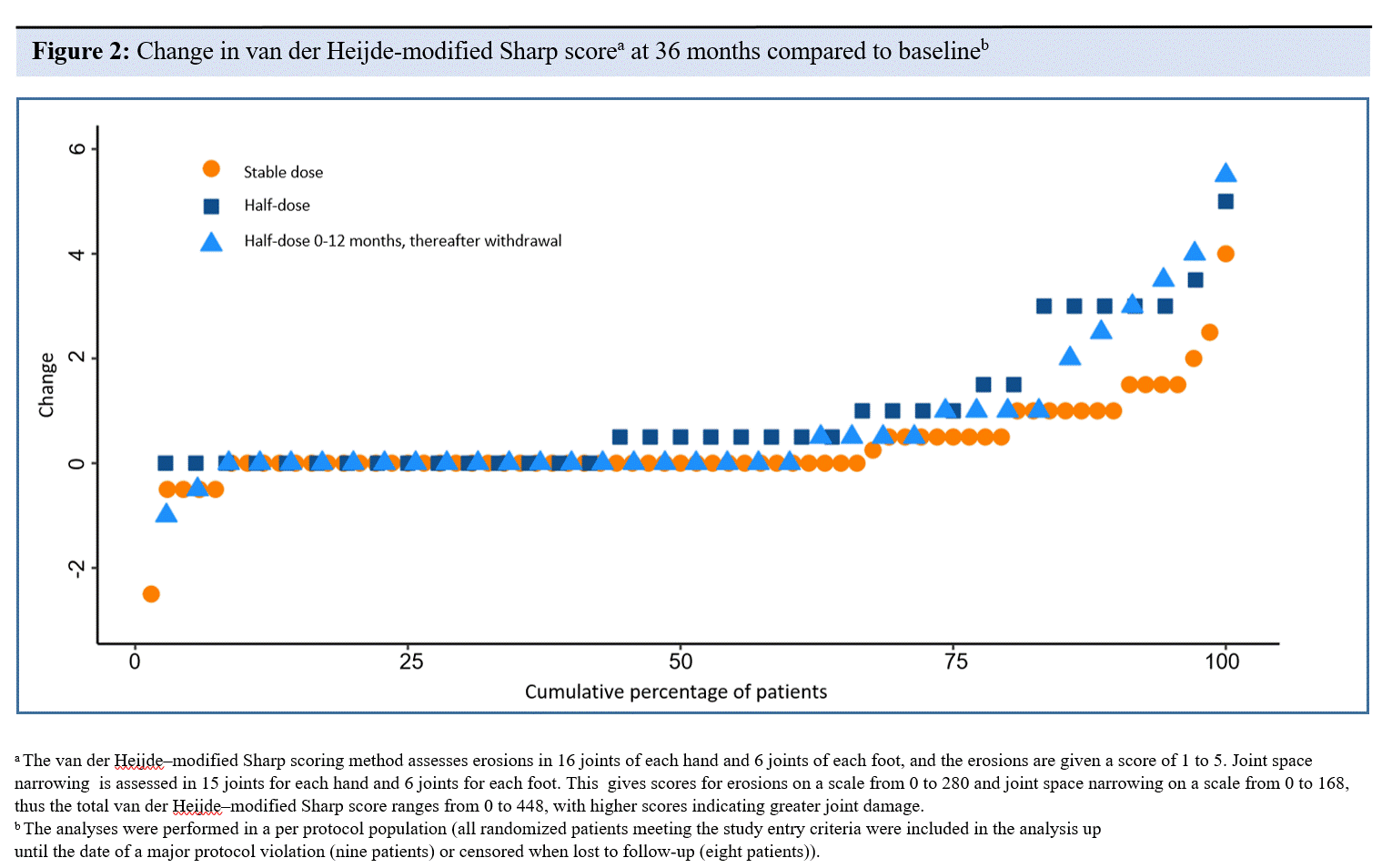
Results: Of 156 patients who received the allocated treatment strategy, 139 patients completed 3-years follow-up without major protocol violation. Mean baseline methotrexate dose/week was 19.0 mg in the stable group, 19.7 mg in the half-dose group, and 19.5 mg in the withdrawal group, and mean DAS at baseline was 0.8 in all groups. 80 % remained flare-free in the stable group, compared with 60 % in the half-dose group (adjusted HR (aHR) for flare 2.7 (95% CI: 1.3 to 5.5)), and 38 % in the tapering to withdrawal group (aHR for flare 4.3 (2.2 to 8.3)) (Figure 1). The aHR was 1.6 (0.8 to 3.0) in the tapering to withdrawal group compared to the half-dose group. A majority were in remission at 1, 2 and 3 years (Table), with the only significant group difference for ACR/EULAR Boolean remission at 3 years, with risk difference for tapering to withdrawal vs stable dose -25% (-45 to -6). Median (IQR) change in vdHSS after 3 years was 0.0 (0-0.5) in the stable group, 0.5 (0.0-1.3) in the half-dose group, and 0.0 (0.0-1.0) in the tapering to withdrawal group (Figure 2), with statistical significant difference between stable and half-dose group, p< 0.01. Sensitivity analyses in the full analysis population gave similar results.
Conclusion: These 3-year data show that 38% of patients in the tapering to withdrawal arm achieved long-term drug-free remission, indicating that this is a realistic option for some RA patients in sustained remission. The two tapering strategies were associated with an increased risk of flares compared to full-dose csDMARD, and the half-dose group had more radiographic change. However, there were no differences in DAS-remission at the end of the study period. Further research identifying prognostic factors for successful tapering is needed.
.gif)
.gif)
K. Kjørholt: None; N. Sundlisæter: None; A. Aga: AbbVie, 2, 6, Eli Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6; J. Sexton: None; I. Olsen: None; H. Fremstad: None; C. Spada: UCB, 12, Advisory Board; T. Madland: Boehringer, 6; C. Høili: None; G. Bakland: UCB, 2; Å. Lexberg: None; I. Hansen: None; I. Hansen: None; H. Haukland: UCB, 12, Advisory Board; M. Ljoså: AbbVie/Abbott, 1, 12, Advisory board fee; E. Moholt: None; T. Uhlig: Galapagos, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB, 2, 6; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; D. Solomon: CorEvitas, 5, Janssen, 5, Moderna, 5, Novartis, 5; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; E. Haavardsholm: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Eli Lilly, 2, Gilead, 2, Pfizer, 6, UCB, 6; S. Lillegraven: Boehringer-Ingelheim, 5.
Background/Purpose: Tapering of disease-modifying antirheumatic drugs (DMARDs) to achieve drug-free remission is a potential goal for the growing group of patients with rheumatoid arthritis (RA) in remission, although long-term effects of such strategies remain unclear. The purpose of this study was to compare the 3-year clinical and radiographic outcomes of three conventional synthetic DMARD (csDMARD) treatment strategies (continued stable treatment, half-dose treatment and tapering to withdrawal) among patients in sustained RA remission.
Methods: ARCTIC REWIND was a randomized, multicenter, open-label, clinical trial enrolling 160 RA patients in sustained remission for ≥1 year on stable csDMARD therapy (Lillegraven et al. JAMA 2021). Patients were randomized 2:1:1 to stable csDMARDs, half-dose csDMARDs, or half-dose csDMARDs for one year followed by withdrawal of all csDMARDs. The primary endpoint was absence of disease activity flare over 3 years. A flare was defined as a combination of DAS >1.6, an increase in DAS ≥0.6 units and ≥2 swollen joints, or if the physician and patient agreed that a clinically significant flare had occurred. Full-dose csDMARD treatment was reinstated upon flare. Secondary endpoints included remission (DAS, ACR/EULAR Boolean), and the 3-year change in radiographic joint damage (van der Heijde-Sharp score, vdHSS). Data were analyzed using Kaplan-Meier, Mann-Whitney test, Cox and mixed effect logistic regression, with stratification or adjustment for study center.
Results: Of 156 patients who received the allocated treatment strategy, 139 patients completed 3-years follow-up without major protocol violation. Mean baseline methotrexate dose/week was 19.0 mg in the stable group, 19.7 mg in the half-dose group, and 19.5 mg in the withdrawal group, and mean DAS at baseline was 0.8 in all groups. 80 % remained flare-free in the stable group, compared with 60 % in the half-dose group (adjusted HR (aHR) for flare 2.7 (95% CI: 1.3 to 5.5)), and 38 % in the tapering to withdrawal group (aHR for flare 4.3 (2.2 to 8.3)) (Figure 1). The aHR was 1.6 (0.8 to 3.0) in the tapering to withdrawal group compared to the half-dose group. A majority were in remission at 1, 2 and 3 years (Table), with the only significant group difference for ACR/EULAR Boolean remission at 3 years, with risk difference for tapering to withdrawal vs stable dose -25% (-45 to -6). Median (IQR) change in vdHSS after 3 years was 0.0 (0-0.5) in the stable group, 0.5 (0.0-1.3) in the half-dose group, and 0.0 (0.0-1.0) in the tapering to withdrawal group (Figure 2), with statistical significant difference between stable and half-dose group, p< 0.01. Sensitivity analyses in the full analysis population gave similar results.
Conclusion: These 3-year data show that 38% of patients in the tapering to withdrawal arm achieved long-term drug-free remission, indicating that this is a realistic option for some RA patients in sustained remission. The two tapering strategies were associated with an increased risk of flares compared to full-dose csDMARD, and the half-dose group had more radiographic change. However, there were no differences in DAS-remission at the end of the study period. Further research identifying prognostic factors for successful tapering is needed.
.gif)
.gif)

K. Kjørholt: None; N. Sundlisæter: None; A. Aga: AbbVie, 2, 6, Eli Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6; J. Sexton: None; I. Olsen: None; H. Fremstad: None; C. Spada: UCB, 12, Advisory Board; T. Madland: Boehringer, 6; C. Høili: None; G. Bakland: UCB, 2; Å. Lexberg: None; I. Hansen: None; I. Hansen: None; H. Haukland: UCB, 12, Advisory Board; M. Ljoså: AbbVie/Abbott, 1, 12, Advisory board fee; E. Moholt: None; T. Uhlig: Galapagos, 2, 6, Lilly, 2, 6, Novartis, 2, 6, Pfizer, 2, 6, UCB, 2, 6; T. Kvien: AbbVie/Abbott, 1, 2, 6, Bristol-Myers Squibb(BMS), 5, Galapagos, 2, 5, Gilead, 2, grunenthal, 6, Janssen, 2, 6, Novartis, 5, Pfizer, 2, 5, sandoz, 2, 6, UCB, 2, 5, 6; D. Solomon: CorEvitas, 5, Janssen, 5, Moderna, 5, Novartis, 5; D. van der Heijde: AbbVie, 2, Bayer, 2, BMS, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, GSK, 2, Imaging Rheumatology BV, 12, Director, Janssen, 2, Novartis, 2, Pfizer, 2, Takeda, 2, UCB Pharma, 2; E. Haavardsholm: AbbVie/Abbott, 2, Boehringer-Ingelheim, 2, Eli Lilly, 2, Gilead, 2, Pfizer, 6, UCB, 6; S. Lillegraven: Boehringer-Ingelheim, 5.



