Poster Session A
Vasculitis
Session: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
0682: Avacopan in ANCA-associated Vasculitis Received Intensified Induction Therapy with Cyclophosphamide Plus Rituximab – Retrospective Case Serial of 12 Patients
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- GA
Gunter Assmann, PhD (he/him/his)
RUB University Hospital Minden JWK
Porta Westfalica, GermanyDisclosure information not submitted.
Abstract Poster Presenter(s)
Gunter Assmann1, Christoph Rittich1, Frank Neumann2, Udo Kellner3, Ryszard Turkiewicz4, Joerg Radermacher5, Peter Lamprecht6 and Björn Tampe7, 1RUB University Hospital Minden - Rheumatology, Minden, Germany, 2Jose Carreras Center Saarland, Homburg, Germany, 3RUB University Hospital Minden - Pathology, Minden, Germany, 4RUB University Hospital Minden - Pulmonology, Minden, Germany, 5RUB University Hospital Minden - Nephrology, Minden, Germany, 6University Hospital Luebeck - Schleswig-Holstein, Luebeck, Germany, 7University Clinic department Goettingen - Nephrology, Goettingen, Germany
Background/Purpose: The orally administered C5a receptor inhibitor avacopan is approved for the therapy of ANCA-associated vasculitis (AAV) in combination with rituximab or cyclophosphamide and shows significant steroid sparing effect as recently published [1]. In severe disease manifestations, preferentially with multiple organ involvement, combined immunochemotherapy of cyclophosphamide plus rituximab can be considered using protocols analogous to the RITUXIVAS study [2] as well as to CycLowVas study [3]. However, there is no experience with the implementation of avacopan in this therapeutic setting.
Methods: Here we present the case serial of 12 patients with AAV, all of whom had severe renal and pulmonary involvement and received induction therapy with high-dose glucocorticoids and cyclophosphamide in combination with rituximab. In addition, patients received avacopan for rapid steroid reduction/slowing down later in the course (from week 2-6), following the above mentioned study [1] in which up to 20mg prednisolone equivalent was given in addition to avacopan as needed. Patients` characteristics are shown in Table 1.All patients were suffering from kidney and lung manifestations and additionally at least one more organ involvement of nervous system, HNO tract, eye, joints or gastrointestinal tract. Three of them had previous courses of cyclophosphamide due to relapsing disease. All patients were treated between February 2022 and June 2023 in the University Hospital Minden (n=10), University Clinic department of Goettingen (n=1), and the Hospital of Holstein-University in Luebeck; the mean observation time was 44.1 weeks (8-72); supportive treatment including anti-infective prophylaxis were administered according to standard treatment regimes.
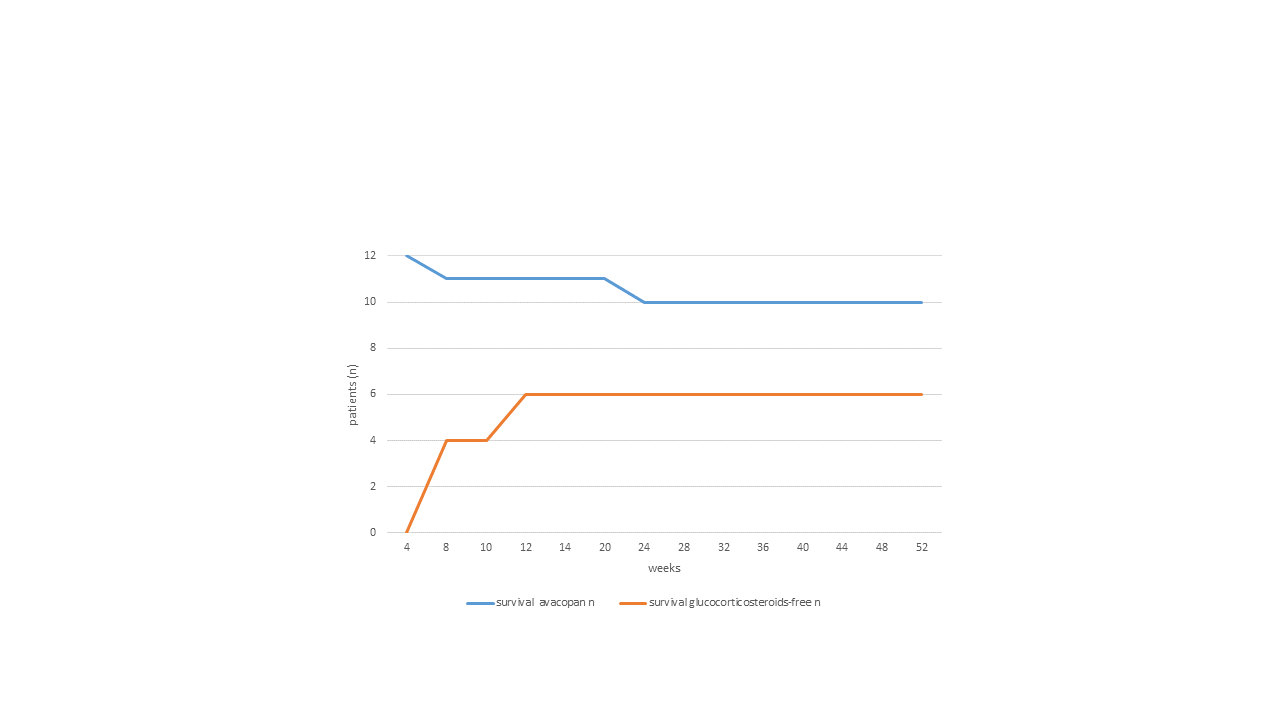
Results: Avacopan were stopped in 2 patients, one due to relapsing disease in week 24, and one due to infectious complication in week 4; the remaining 10 patients on avacopan achieved stable remission of AVV, in 6 cases even with successful discontinuation of glucocorticosteroids on average after 11.8 weeks (from 6 to 20; figure 1). 4-6 months after induction therapy, patients received rituximab maintenance therapy in accordance with guidelines (n=8).
Conclusion: Avacopan appears to favor steroid tapering in severe courses of AAV on combined therapy with cyclophosphamide plus rituximab. However, due to the small number of cases and the retrospective evaluation, the results have to be confirmed by studies with larger number of cases.
[References: [1] Jayne DRW et al, NEJM, 2021. [2] Jones RB et al, NEJM, 2010. [3] McAdoo SP et al, Nephrol Dial Transplant, 2018]
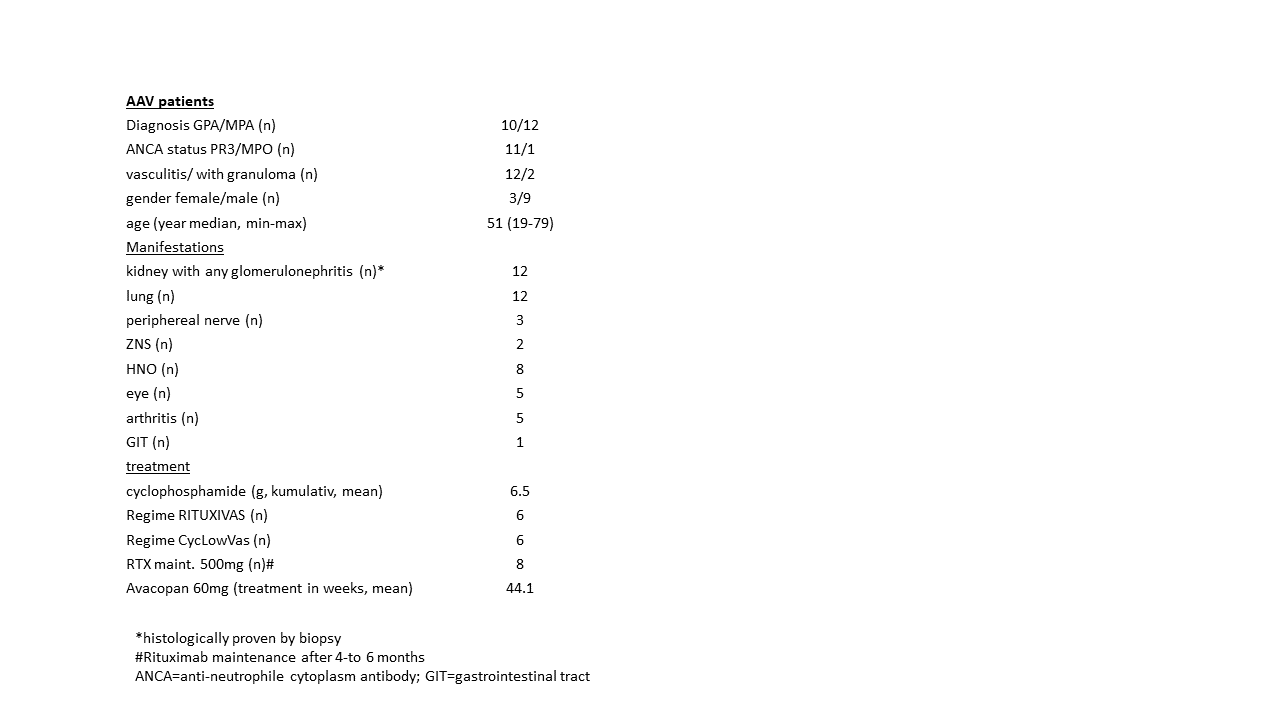
G. Assmann: AbbVie/Abbott, 2, 5, 6, AstraZeneca, 2, Boehringer-Ingelheim, 2, 6, Novartis, 5, UCB, 1; C. Rittich: None; F. Neumann: None; U. Kellner: None; R. Turkiewicz: None; J. Radermacher: None; P. Lamprecht: AstraZeneca, 2, GlaxoSmithKlein(GSK), 2, 5, Novartis, 2, Vifor, 2, 5; B. Tampe: Vifor, 6.
Background/Purpose: The orally administered C5a receptor inhibitor avacopan is approved for the therapy of ANCA-associated vasculitis (AAV) in combination with rituximab or cyclophosphamide and shows significant steroid sparing effect as recently published [1]. In severe disease manifestations, preferentially with multiple organ involvement, combined immunochemotherapy of cyclophosphamide plus rituximab can be considered using protocols analogous to the RITUXIVAS study [2] as well as to CycLowVas study [3]. However, there is no experience with the implementation of avacopan in this therapeutic setting.
Methods: Here we present the case serial of 12 patients with AAV, all of whom had severe renal and pulmonary involvement and received induction therapy with high-dose glucocorticoids and cyclophosphamide in combination with rituximab. In addition, patients received avacopan for rapid steroid reduction/slowing down later in the course (from week 2-6), following the above mentioned study [1] in which up to 20mg prednisolone equivalent was given in addition to avacopan as needed. Patients` characteristics are shown in Table 1.All patients were suffering from kidney and lung manifestations and additionally at least one more organ involvement of nervous system, HNO tract, eye, joints or gastrointestinal tract. Three of them had previous courses of cyclophosphamide due to relapsing disease. All patients were treated between February 2022 and June 2023 in the University Hospital Minden (n=10), University Clinic department of Goettingen (n=1), and the Hospital of Holstein-University in Luebeck; the mean observation time was 44.1 weeks (8-72); supportive treatment including anti-infective prophylaxis were administered according to standard treatment regimes.
Results: Avacopan were stopped in 2 patients, one due to relapsing disease in week 24, and one due to infectious complication in week 4; the remaining 10 patients on avacopan achieved stable remission of AVV, in 6 cases even with successful discontinuation of glucocorticosteroids on average after 11.8 weeks (from 6 to 20; figure 1). 4-6 months after induction therapy, patients received rituximab maintenance therapy in accordance with guidelines (n=8).
Conclusion: Avacopan appears to favor steroid tapering in severe courses of AAV on combined therapy with cyclophosphamide plus rituximab. However, due to the small number of cases and the retrospective evaluation, the results have to be confirmed by studies with larger number of cases.
[References: [1] Jayne DRW et al, NEJM, 2021. [2] Jones RB et al, NEJM, 2010. [3] McAdoo SP et al, Nephrol Dial Transplant, 2018]
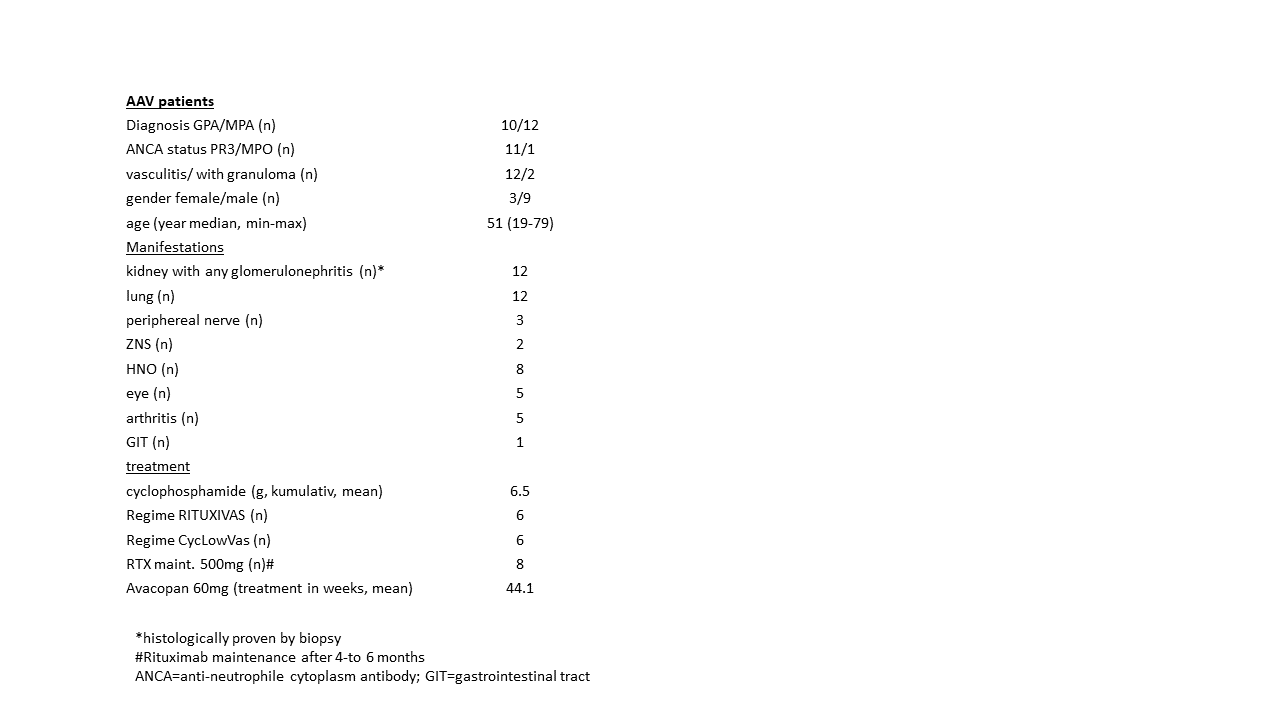
Table 1. Characteristics of ANCA-associated vasculitis patients (n=12)

Figure 1: Survival of avacopan treatment and corresponding glucocorticosteroid free in ANCA-associated vasculitis patients (n=12) over 52 weeks
G. Assmann: AbbVie/Abbott, 2, 5, 6, AstraZeneca, 2, Boehringer-Ingelheim, 2, 6, Novartis, 5, UCB, 1; C. Rittich: None; F. Neumann: None; U. Kellner: None; R. Turkiewicz: None; J. Radermacher: None; P. Lamprecht: AstraZeneca, 2, GlaxoSmithKlein(GSK), 2, 5, Novartis, 2, Vifor, 2, 5; B. Tampe: Vifor, 6.



