Poster Session A
Rheumatoid arthritis (RA)
Session: (0423–0459) RA – Treatment Poster I
0429: Direct and Indirect Effects of Upadacitinib or Adalimumab on Pain in Rheumatoid Arthritis: Results from a Randomized Phase 3 Study
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- PT
Peter Taylor, PhD, FRCP, MA
University of Oxford
Oxford, United KingdomDisclosure information not submitted.
Abstract Poster Presenter(s)
Peter C. Taylor1, David Walsh2, Tsutomu Takeuchi3, Bruno Fautrel4, Janet Pope5, Andrew Garrison6, Yanna Song6, Sara K. Penn6, Ralph Lippe7, Diane Caballero8 and Arthur Kavanaugh9, 1Nuffield Department of Orthopedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom, 2University of Nottingham, Nottingham, United Kingdom, 3Keio University School of Medicine and Saitama Medical University, Tokyo, Japan, 4Sorbonne Université APHP, Paris, France, 5University of Western Ontario, London, ON, Canada, 6AbbVie, Inc., North Chicago, IL, 7AbbVie Deutschland GmbH & Co. KG, Wiesbaden, Germany, 8AbbVie, Inc., Chicago, IL, 9University of California San Diego, School of Medicine, Riverside, CA
Background/Purpose: Rapid and sustained pain control is an important goal for patients (pts) with rheumatoid arthritis (RA). Control of inflammation in RA does not always eliminate pain, which can have multifactorial causes. Our objective was to assess direct and indirect (ie, by inflammation surrogates) effects of treatment with upadacitinib (UPA) or adalimumab (ADA) vs placebo (PBO) on pain in pts with RA.
Methods: Period 1 of SELECT-COMPARE was a 48-week, randomized, double-blind, phase 3 study with a 26-week PBO-controlled period in pts with RA who had active disease despite methotrexate treatment. Adults (≥18 years) on stable background methotrexate were randomized 2:2:1 to UPA 15 mg once daily, PBO, or ADA 40 mg every other week. Pts with an insufficient response (< 20% improvement in tender joint count [TJC] or swollen joint count [SJC] at week 14, 18, and 22 or if Clinical Disease Activity Index was >10 at week 26) were rescued from PBO to UPA, UPA to ADA, or ADA to UPA; all PBO pts who were not rescued were switched to UPA at week 26. Observed case analysis was performed for change from baseline in Patient’s Global Assessment (PtGA) of pain (1–100 mm). A multiple mediator analysis1 for effect of UPA vs PBO and ADA vs PBO on pain assessed as PtGA of pain or TJC28 was conducted using observed case analysis for week 2 and 12 analyses and last observation carried forward for data after rescue for the week 26 analysis. Indirect effect of treatment on pain was assessed based on ESR, CRP, and SJC28.
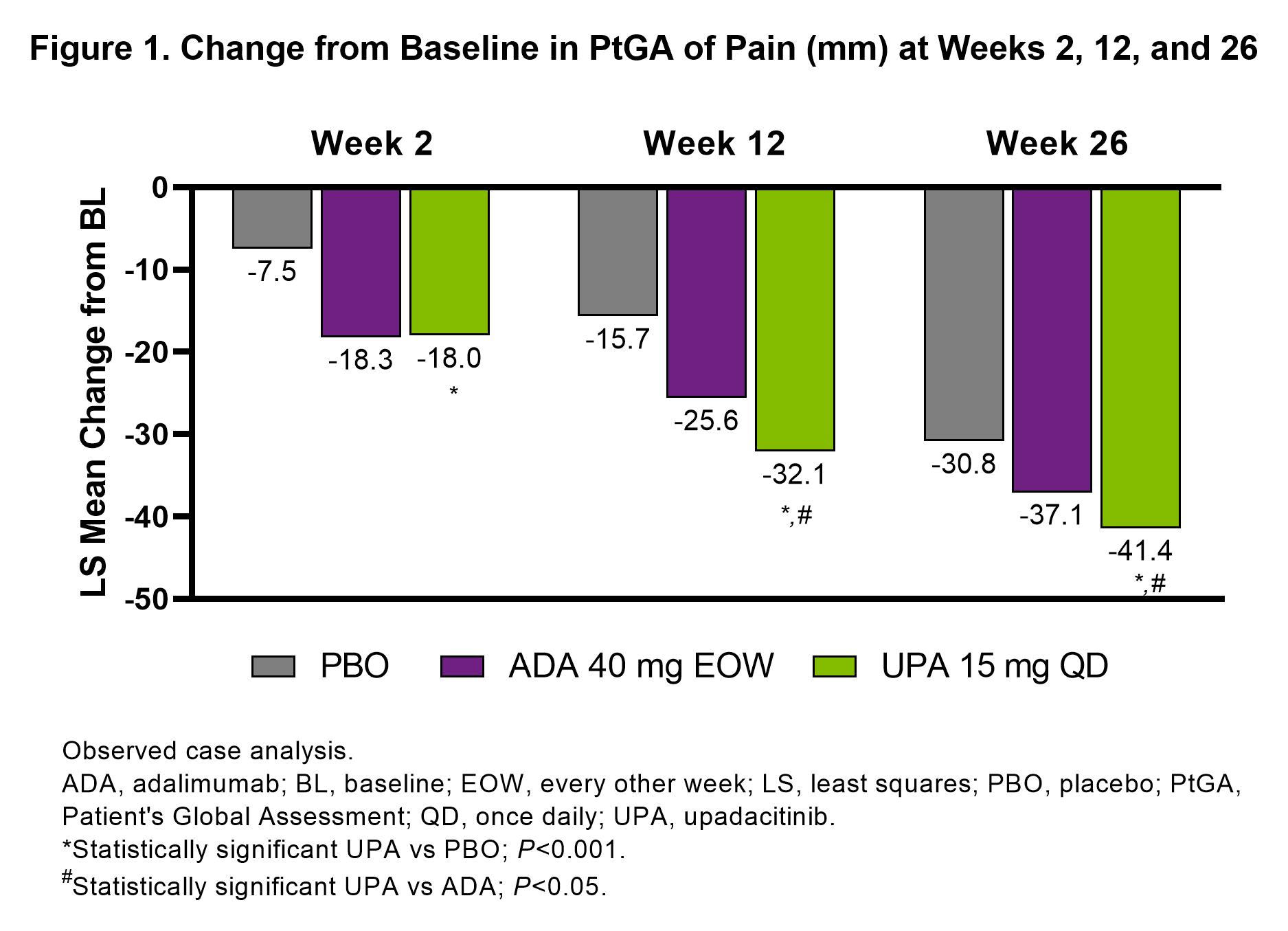
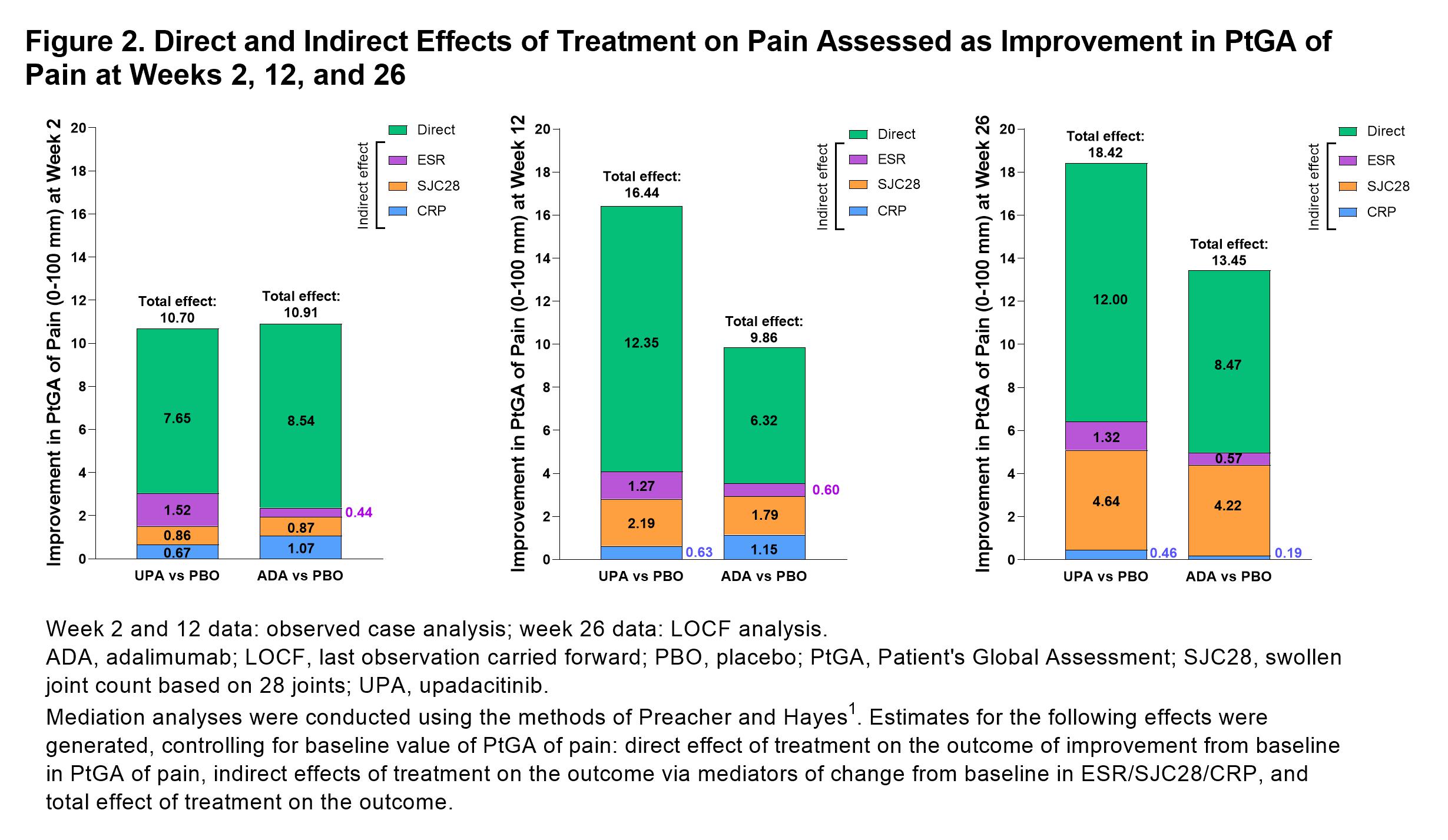
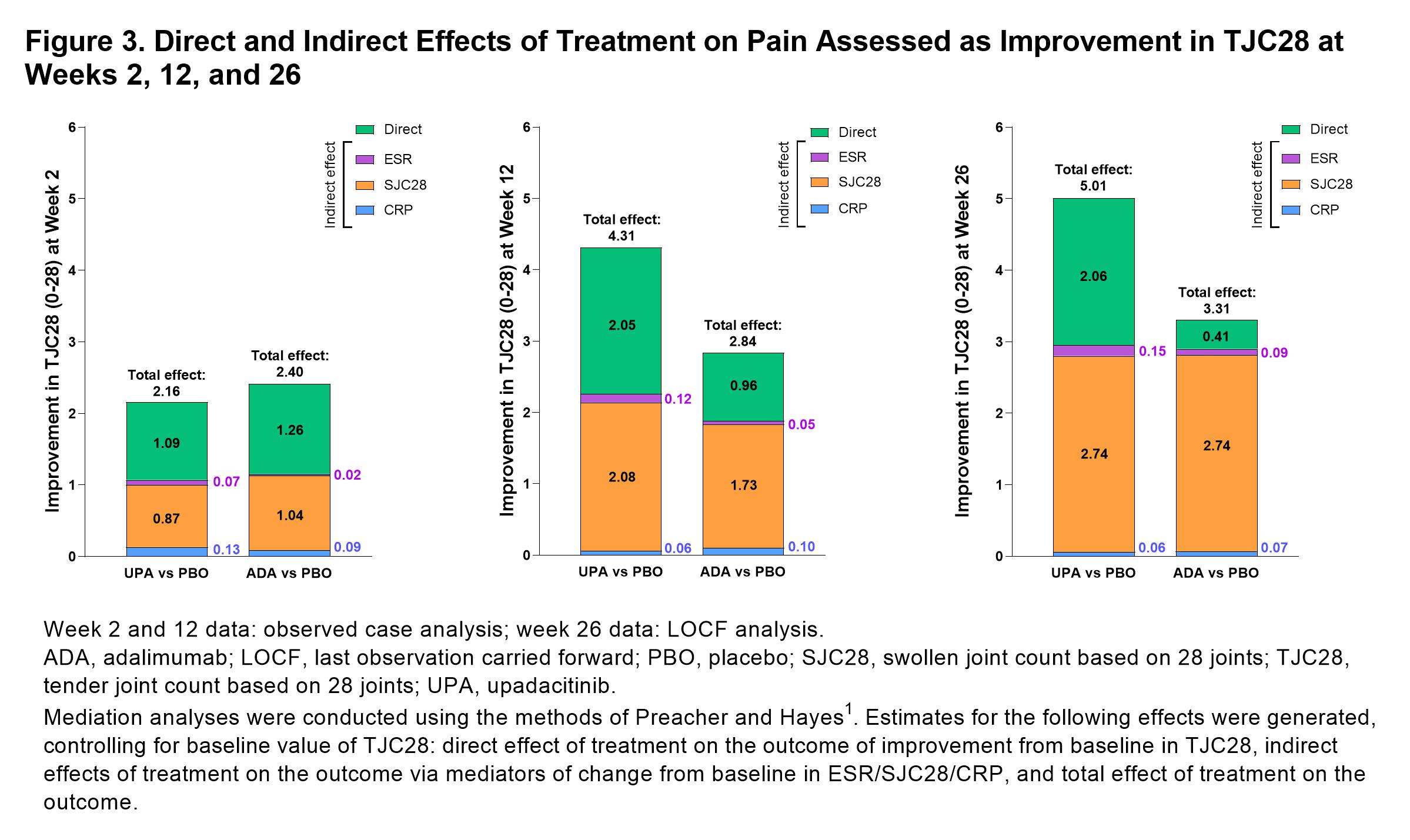
Results: 1629 pts were included in this analysis (UPA, n=651, PBO, n=651, ADA, n=327).PtGA of pain significantly improved with UPA vs PBO from baseline to week 2 (–18.0 [n=639] vs –7.5 [n=624]; P< 0.001), week 12 (–32.1 [n=614] vs –15.7 [n=616]; P< 0.001), and week 26 (–41.4 [n=478] vs –30.8 [n=305]; P< 0.001; Figure 1). Improvements in PtGA of pain were also observed with ADA vs PBO at weeks 2 (–18.3 [n=318]), 12 (–25.6 [n=307]), and 26 (–37.1 [n=212]; Figure 1). Total and direct effects on PtGA of pain improvement were significantly greater (both P< 0.001) with UPA vs PBO as early as week 2 (Figure 2) and increased at weeks 12 and 26 (all P< 0.001). Total and direct effects on PtGA of pain improvement were also observed with ADA vs PBO (Figure 2). Improvement in pain assessed as TJC28 was also significantly greater (all P< 0.05) with UPA and ADA vs PBO at weeks 2, 12, and 26; SJC28 showed the greatest indirect effect on pain assessed as TJC28 at weeks 12 and 26 (Figure 3). UPA and ADA vs PBO showed similar indirect contributions to improvement in PtGA of pain and TJC28 at weeks 2, 12, and 26, whereas UPA showed a greater direct effect on PtGA of pain and TJC28 at weeks 12 and 26 than ADA (Figures 2 and 3).
Conclusion: Both UPA and ADA resulted in rapid and significant improvements in pain vs PBO as early as week 2 in patients with RA. Indirect effects on PtGA or TJC28 pain improvement were similar between UPA and ADA at all time points; however, direct effect on pain was up to two times greater with UPA vs ADA at weeks 12 and 26. These results suggest UPA may be more effective in control of pain, which may be due to either inflammatory or non-inflammatory mechanisms, than ADA in RA.
Reference:1Preacher, KJ & Hayes, AF. Behavior Research Methods,2008;40(3), 879–891.
P. Taylor: AbbVie, 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 5, Gilead Sciences, 2, GSK, 2, Janssen, 2, Nordic Pharma, 2, Pfizer Inc, 2, Sanofi, 2, UCB, 2; D. Walsh: AbbVie, 2, Contura International A/S, 2, Eli Lilly, 5, GlaxoSmithKline Research & Development Limited, 5, Orion Corporation, 5, Pfizer, 5; T. Takeuchi: AbbVie, 2, 5, 6, AYUMI, 5, Bristol-Myers Squibb, 6, Chugai, 2, 5, 6, Daiichi Sankyo, 5, Eisai, 5, 6, Eli Lilly Japan, 2, 6, Gilead, 2, 6, Janssen, 6, Mitsubishi-Tanabe, 2, 5, 6, ONO, 5, Pfizer Japan, 6, Taiho, 2; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2; J. Pope: AbbVie, 1, 2; A. Garrison: AbbVie, 3, 11; Y. Song: AbbVie, 3, 11; S. Penn: AbbVie, 3, 11; R. Lippe: AbbVie, 3, 11; D. Caballero: AbbVie, 3, 11; A. Kavanaugh: AbbVie, 1, 2, Amgen, 1, 2, BMS, 1, 2, Eli Lilly, 1, 2, Novartis, 1, 2, Pfizer, 1, 2, UCB, 1, 2.
Background/Purpose: Rapid and sustained pain control is an important goal for patients (pts) with rheumatoid arthritis (RA). Control of inflammation in RA does not always eliminate pain, which can have multifactorial causes. Our objective was to assess direct and indirect (ie, by inflammation surrogates) effects of treatment with upadacitinib (UPA) or adalimumab (ADA) vs placebo (PBO) on pain in pts with RA.
Methods: Period 1 of SELECT-COMPARE was a 48-week, randomized, double-blind, phase 3 study with a 26-week PBO-controlled period in pts with RA who had active disease despite methotrexate treatment. Adults (≥18 years) on stable background methotrexate were randomized 2:2:1 to UPA 15 mg once daily, PBO, or ADA 40 mg every other week. Pts with an insufficient response (< 20% improvement in tender joint count [TJC] or swollen joint count [SJC] at week 14, 18, and 22 or if Clinical Disease Activity Index was >10 at week 26) were rescued from PBO to UPA, UPA to ADA, or ADA to UPA; all PBO pts who were not rescued were switched to UPA at week 26. Observed case analysis was performed for change from baseline in Patient’s Global Assessment (PtGA) of pain (1–100 mm). A multiple mediator analysis1 for effect of UPA vs PBO and ADA vs PBO on pain assessed as PtGA of pain or TJC28 was conducted using observed case analysis for week 2 and 12 analyses and last observation carried forward for data after rescue for the week 26 analysis. Indirect effect of treatment on pain was assessed based on ESR, CRP, and SJC28.
Results: 1629 pts were included in this analysis (UPA, n=651, PBO, n=651, ADA, n=327).PtGA of pain significantly improved with UPA vs PBO from baseline to week 2 (–18.0 [n=639] vs –7.5 [n=624]; P< 0.001), week 12 (–32.1 [n=614] vs –15.7 [n=616]; P< 0.001), and week 26 (–41.4 [n=478] vs –30.8 [n=305]; P< 0.001; Figure 1). Improvements in PtGA of pain were also observed with ADA vs PBO at weeks 2 (–18.3 [n=318]), 12 (–25.6 [n=307]), and 26 (–37.1 [n=212]; Figure 1). Total and direct effects on PtGA of pain improvement were significantly greater (both P< 0.001) with UPA vs PBO as early as week 2 (Figure 2) and increased at weeks 12 and 26 (all P< 0.001). Total and direct effects on PtGA of pain improvement were also observed with ADA vs PBO (Figure 2). Improvement in pain assessed as TJC28 was also significantly greater (all P< 0.05) with UPA and ADA vs PBO at weeks 2, 12, and 26; SJC28 showed the greatest indirect effect on pain assessed as TJC28 at weeks 12 and 26 (Figure 3). UPA and ADA vs PBO showed similar indirect contributions to improvement in PtGA of pain and TJC28 at weeks 2, 12, and 26, whereas UPA showed a greater direct effect on PtGA of pain and TJC28 at weeks 12 and 26 than ADA (Figures 2 and 3).
Conclusion: Both UPA and ADA resulted in rapid and significant improvements in pain vs PBO as early as week 2 in patients with RA. Indirect effects on PtGA or TJC28 pain improvement were similar between UPA and ADA at all time points; however, direct effect on pain was up to two times greater with UPA vs ADA at weeks 12 and 26. These results suggest UPA may be more effective in control of pain, which may be due to either inflammatory or non-inflammatory mechanisms, than ADA in RA.
Reference:1Preacher, KJ & Hayes, AF. Behavior Research Methods,2008;40(3), 879–891.

Figure 1. Change from Baseline in PtGA of Pain (mm) at Weeks 2, 12, and 26

Figure 2. Direct and Indirect Effects of Treatment on Pain Assessed as Improvement in PtGA of Pain at Weeks 2, 12, and 26

Figure 3. Direct and Indirect Effects of Treatment on Pain Assessed as Improvement in TJC28 at Weeks 2, 12, and 26
P. Taylor: AbbVie, 2, Biogen, 2, Eli Lilly, 2, Fresenius, 2, Galapagos, 2, 5, Gilead Sciences, 2, GSK, 2, Janssen, 2, Nordic Pharma, 2, Pfizer Inc, 2, Sanofi, 2, UCB, 2; D. Walsh: AbbVie, 2, Contura International A/S, 2, Eli Lilly, 5, GlaxoSmithKline Research & Development Limited, 5, Orion Corporation, 5, Pfizer, 5; T. Takeuchi: AbbVie, 2, 5, 6, AYUMI, 5, Bristol-Myers Squibb, 6, Chugai, 2, 5, 6, Daiichi Sankyo, 5, Eisai, 5, 6, Eli Lilly Japan, 2, 6, Gilead, 2, 6, Janssen, 6, Mitsubishi-Tanabe, 2, 5, 6, ONO, 5, Pfizer Japan, 6, Taiho, 2; B. Fautrel: AbbVie, 2, BMS, 2, Chugai, 2, Fresenius Kabi, 2, Galapagos, 2, Lilly, 2, Medac, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Sobi, 2, UCB, 2; J. Pope: AbbVie, 1, 2; A. Garrison: AbbVie, 3, 11; Y. Song: AbbVie, 3, 11; S. Penn: AbbVie, 3, 11; R. Lippe: AbbVie, 3, 11; D. Caballero: AbbVie, 3, 11; A. Kavanaugh: AbbVie, 1, 2, Amgen, 1, 2, BMS, 1, 2, Eli Lilly, 1, 2, Novartis, 1, 2, Pfizer, 1, 2, UCB, 1, 2.



