Poster Session A
Osteoarthritis (OA) and related disorders
Session: (0308–0324) Osteoarthritis – Clinical Poster I
0314: Lack of Systemic Effects from Intra-articular XT-150 for the Treatment of Moderate to Severe Pain Due to OA of the Knee: Safety Results from a Phase 2 Trial
Sunday, November 12, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- HR
Howard Rutman, MD, MBA
Xalud Therapeutics
New York, NY, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Leonard Kapural1, Stephen D. Collins2, Eric Grigsby3, Mark McBride4, Jayson Rieger5, Morgan Stokes6, Howard Rutman6 and Flavia Cicuttini7, 1Center for Clinical Research, Winston-Salem, NC, 2Xalud Therapeutics, Inc., Chicago, IL, 3Neurovations Clinical Research, Napa, CA, 4Instat Clinical Research, Chatham, NJ, 5PBM Capital, Charlottesville, VA, 6Xalud Therapeutics, Inc., Berkeley, CA, 7Monash University, Melbourne, Australia
Background/Purpose: This study (NCT04124042) was designed to assess the safety, tolerability, and efficacy of XT-150, a non-viral gene therapy, in adult participants with moderate to severe pain due to osteoarthritis (OA) of the knee. XT-150 is a plasmid designed to locally express a proprietary version of the anti-inflammatory cytokine, interleukin (IL)-10. IL-10 has been shown to play a key role in pain reversal but has proven a challenging therapeutic candidate due to its short half-life.1 We hypothesize that local expression of IL-10 via gene therapy may be a more appropriate route of administration for sustained effects and has shown acceptable safety profiles and no-anti-IL-10 antibodies in previous clinical and pre-clinical studies.2
Methods: We describe safety results from a two-stage double-blind Phase 2 study. Stage A compared 2 active doses of XT-150 to a placebo (phosphate-buffered saline) control arm. Stage B was a 6-month follow-up with the option to randomly receive a single injection of XT-150 at one of two doses (0.15 or 0.45 mg) between Day 180 and Day 330. All intra-articular (IA) injections of XT-150 or placebo were performed under imaging guidance (Table 1). Participants 45-85 years of age had symptomatic knee OA defined as a WOMAC Pain score of ≥8 (out of 20) and Kellgren-Lawrence grade 2 or 3 at screening. After signing the Informed Consent Form (ICF), all were assessed for safety by physical examination, laboratory assessments, vital signs, adverse events (AEs) and serious AEs (SAEs) throughout the study. All participants (N=286) receiving any amount of XT-150 or placebo were included in the safety analysis. Safety data were summarized using descriptive statistics according to a prespecified statistical analysis plan (SAP).
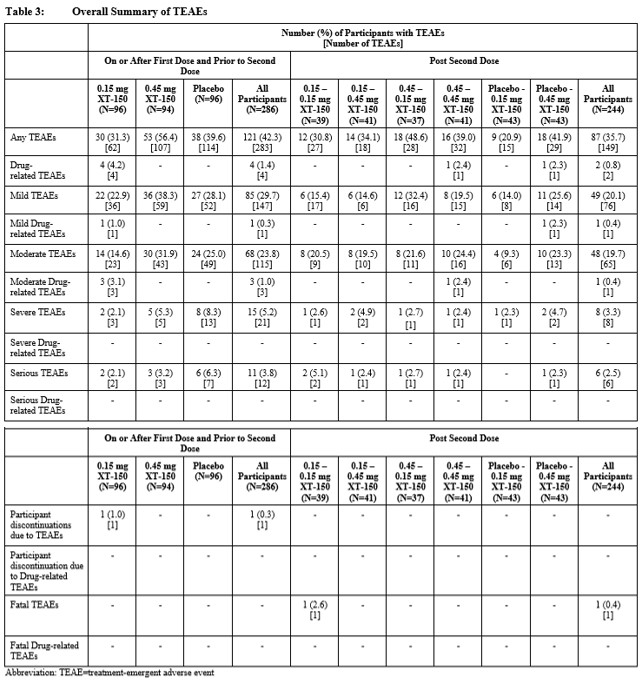
Results: Drug-related AEs in this study were local with no systemic AEs. Local injection drug-related treatment-emergent adverse events (TEAEs) were reported by 6 patients (2%); arthralgia was the most common (Table 2). Overall, XT-150 was generally well tolerated in single and multiple IA injections. No dose-, treatment-, or sequence-related trends were reported for any safety domain for XT-150 (Table 3). The most commonly occurring TEAE for all participants who received active study drug was arthralgia (13% of participants). One unrelated TEAE of Grade 5 intensity, a myocardial infarction, was reported following the second dose in a patient with a history of CVD. SAEs were reported across all arms and treatment sequences with a total of 17 (6%) participants reporting 18 SAEs over the 360-day study period, all of which were considered unrelated to the study treatment. There were no AEs of special interest related to study treatment. Anti-IL-10 antibodies were not detected after single or repeat injections.
Conclusion: Safety data from this study support continued investigation of XT-150 for the management of pain and improvement of physical function in patients with knee OA. The local nature of treatment-related AEs and the absence of treatment-related SAEs support a two-dose regimen of 0.45 mg XT-150 (total dose of 0.9 mg) as safe and well-tolerated for future studies.
1. Kwilasz AJ, et al. Neuropharmacology. 2015;96(Pt A):55-69; 2. Watkins LR, et al. Brain Behav Immun. 2020 Nov;90:155-166.
.jpg)
.jpg)
L. Kapural: Avanos, 1, 5, Gimer, 1, Nalu, 5, Neuralace, 5, Neuros, 1, Nevro, 1, 5, PainTeq, 1, Presidio, 1, Saluda, 5; S. Collins: Xalud Therapeutics, Inc., 1, 4, 8, 11; E. Grigsby: Eli Lilly, 5, Jointstem, 5, Kolon Tissuegene, 5, Medtronic, 2, 5, Neuros, 5, Sollis Therapeutics, 2, Tenex Health Inc., 2, Xalud Therapeutics, 2; M. McBride: None; J. Rieger: Xalud Therapeutics, 4, 8, 10; M. Stokes: Xalud Therapeutics, 3; H. Rutman: Xalud Therapeutics, 3, 11; F. Cicuttini: Xalud Therapeutics, Inc., 5.
Background/Purpose: This study (NCT04124042) was designed to assess the safety, tolerability, and efficacy of XT-150, a non-viral gene therapy, in adult participants with moderate to severe pain due to osteoarthritis (OA) of the knee. XT-150 is a plasmid designed to locally express a proprietary version of the anti-inflammatory cytokine, interleukin (IL)-10. IL-10 has been shown to play a key role in pain reversal but has proven a challenging therapeutic candidate due to its short half-life.1 We hypothesize that local expression of IL-10 via gene therapy may be a more appropriate route of administration for sustained effects and has shown acceptable safety profiles and no-anti-IL-10 antibodies in previous clinical and pre-clinical studies.2
Methods: We describe safety results from a two-stage double-blind Phase 2 study. Stage A compared 2 active doses of XT-150 to a placebo (phosphate-buffered saline) control arm. Stage B was a 6-month follow-up with the option to randomly receive a single injection of XT-150 at one of two doses (0.15 or 0.45 mg) between Day 180 and Day 330. All intra-articular (IA) injections of XT-150 or placebo were performed under imaging guidance (Table 1). Participants 45-85 years of age had symptomatic knee OA defined as a WOMAC Pain score of ≥8 (out of 20) and Kellgren-Lawrence grade 2 or 3 at screening. After signing the Informed Consent Form (ICF), all were assessed for safety by physical examination, laboratory assessments, vital signs, adverse events (AEs) and serious AEs (SAEs) throughout the study. All participants (N=286) receiving any amount of XT-150 or placebo were included in the safety analysis. Safety data were summarized using descriptive statistics according to a prespecified statistical analysis plan (SAP).
Results: Drug-related AEs in this study were local with no systemic AEs. Local injection drug-related treatment-emergent adverse events (TEAEs) were reported by 6 patients (2%); arthralgia was the most common (Table 2). Overall, XT-150 was generally well tolerated in single and multiple IA injections. No dose-, treatment-, or sequence-related trends were reported for any safety domain for XT-150 (Table 3). The most commonly occurring TEAE for all participants who received active study drug was arthralgia (13% of participants). One unrelated TEAE of Grade 5 intensity, a myocardial infarction, was reported following the second dose in a patient with a history of CVD. SAEs were reported across all arms and treatment sequences with a total of 17 (6%) participants reporting 18 SAEs over the 360-day study period, all of which were considered unrelated to the study treatment. There were no AEs of special interest related to study treatment. Anti-IL-10 antibodies were not detected after single or repeat injections.
Conclusion: Safety data from this study support continued investigation of XT-150 for the management of pain and improvement of physical function in patients with knee OA. The local nature of treatment-related AEs and the absence of treatment-related SAEs support a two-dose regimen of 0.45 mg XT-150 (total dose of 0.9 mg) as safe and well-tolerated for future studies.
1. Kwilasz AJ, et al. Neuropharmacology. 2015;96(Pt A):55-69; 2. Watkins LR, et al. Brain Behav Immun. 2020 Nov;90:155-166.
.jpg)
.jpg)

L. Kapural: Avanos, 1, 5, Gimer, 1, Nalu, 5, Neuralace, 5, Neuros, 1, Nevro, 1, 5, PainTeq, 1, Presidio, 1, Saluda, 5; S. Collins: Xalud Therapeutics, Inc., 1, 4, 8, 11; E. Grigsby: Eli Lilly, 5, Jointstem, 5, Kolon Tissuegene, 5, Medtronic, 2, 5, Neuros, 5, Sollis Therapeutics, 2, Tenex Health Inc., 2, Xalud Therapeutics, 2; M. McBride: None; J. Rieger: Xalud Therapeutics, 4, 8, 10; M. Stokes: Xalud Therapeutics, 3; H. Rutman: Xalud Therapeutics, 3, 11; F. Cicuttini: Xalud Therapeutics, Inc., 5.



