Abstract Session
Rheumatoid arthritis (RA)
Session: Abstracts: RA – Etiology & Pathogenesis (2431–2436)
2432: Wnt Signaling Drives Pathogenic Stromal Inflammation in Inflammatory Arthritis
Tuesday, November 14, 2023
2:15 PM - 2:25 PM PT
Location: Room 29A-D

Alisa Mueller, MD, PhD
Fellow
Brigham and Women's Hospital
Brigham and Women's Hospital
Boston, MA, United StatesDisclosure information not submitted.
Presenting Author(s)
Alisa Mueller1, Angela Zou2, Lucy-Jayne Marsh3, Samuel Kemble4, Saba Nayar3, Emily Taylor3, Triin Major3, David Gardner5, Gerald F.M. Watts1, Cassandra Murphy1, Roche Fibroblast Network Consortium6, Adam Croft3, Andrew Filer3, Christopher Buckley7, Kevin Wei8, ilya Korsunsky1, Soumya Raychaudhuri1 and Michael Brenner8, 1Brigham and Women's Hospital, Boston, MA, 2Harvard Medical School, Boston, MA, 3University of Birmingham, Birmingham, United Kingdom, 4University Birmingham, Rugeley, United Kingdom, 5University of Birmingham, Havelock North, New Zealand, 6Roche, Basel, Switzerland, 7University of Oxford, Oxford, United Kingdom, 8Brigham and Women's Hospital and Harvard Medical School, Boston, MA
Background/Purpose: Synovial fibroblasts are a promising therapeutic target in rheumatoid arthritis (RA) where they can adopt inflammatory or destructive phenotypes and directly promote bone and cartilage degradation. In our prior analyses, we found that non-canonical Wnt activation induces robust inflammatory gene expression in these cells. Moreover, two Wnt modulators, RSPO3 and DKK3, are reciprocally expressed and form a transcriptional gradient associated with increased and decreased Wnt activation, respectively. Strikingly, RSPO3 and Wnt activation signatures are enhanced in synovial fibroblasts in RA, suggesting the potential relevance of the Wnt pathway in vivo. Here, we explore the clinical impact of Wnt signaling in mouse models of inflammatory arthritis where we demonstrate that Wnt activation worsens the severity and prolongs the course of arthritis.
Methods: Single-cell RNA sequencing (scRNA-seq) libraries were generated from enzymatically digested, sort-purified CD45- synovial tissue dissected from K/BxN serum recipient mice at the indicated time points. To generate Wnt activation signatures, human RA synovial fibroblasts were stimulated in vitro with a combination of Wnt ligands, doses, and time points, and we performed RNA-seq and differential gene expression analysis. RSPO3 signatures were developed from scRNA-seq data of synovial fibroblasts from the Roche Fibroblast Network Consortium. In the antigen-induced arthritis (AIA) model, C57Bl/6 mice underwent subcutaneous (day -21) and knee intra-articular (day 0) injections of methylated BSA (mBSA) followed by knee intra-articular injections of PBS or Wnt5a (days 2 and 4). Knee diameters were measured using calipers, and synovial samples were collected at day 7 for scRNA-seq.
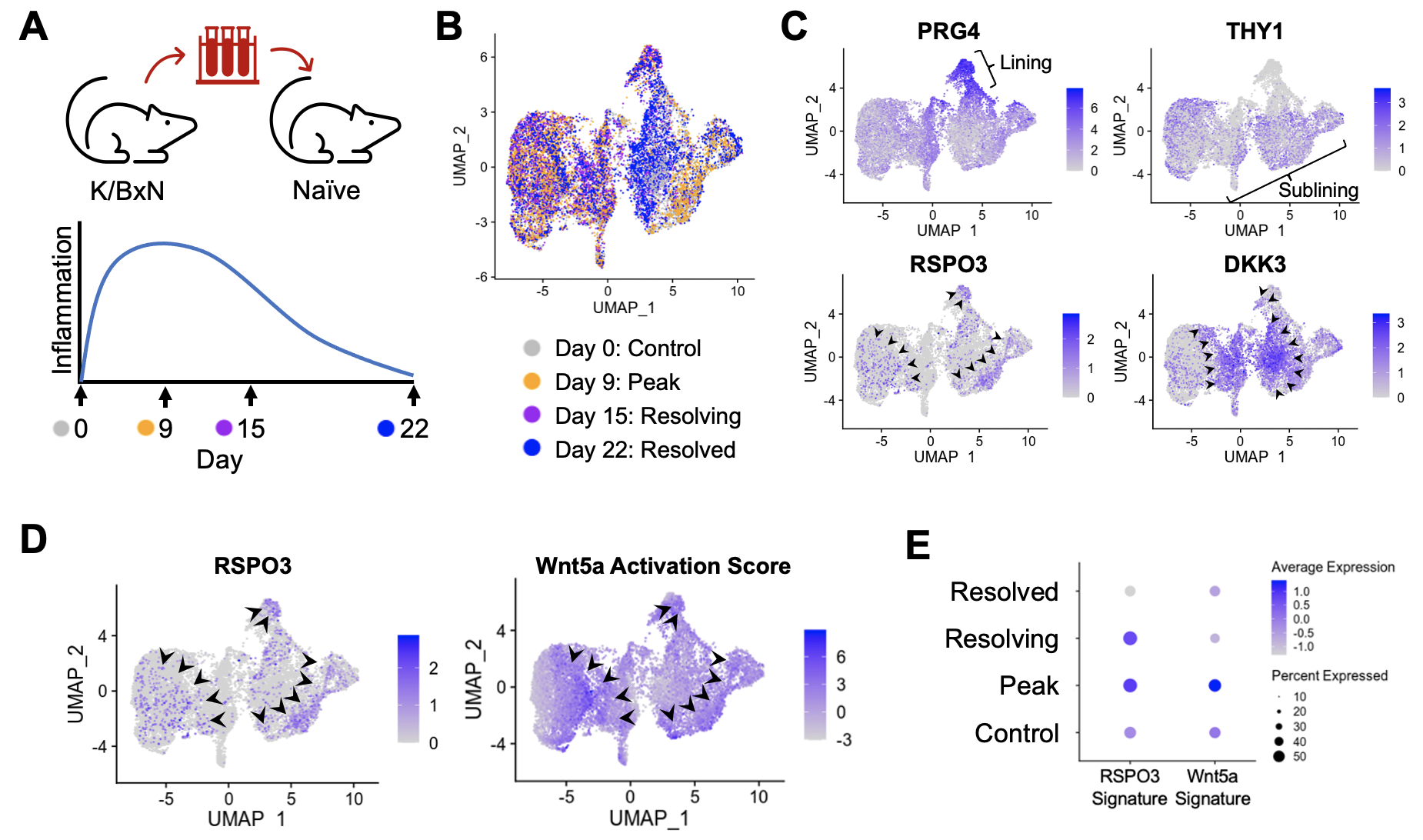
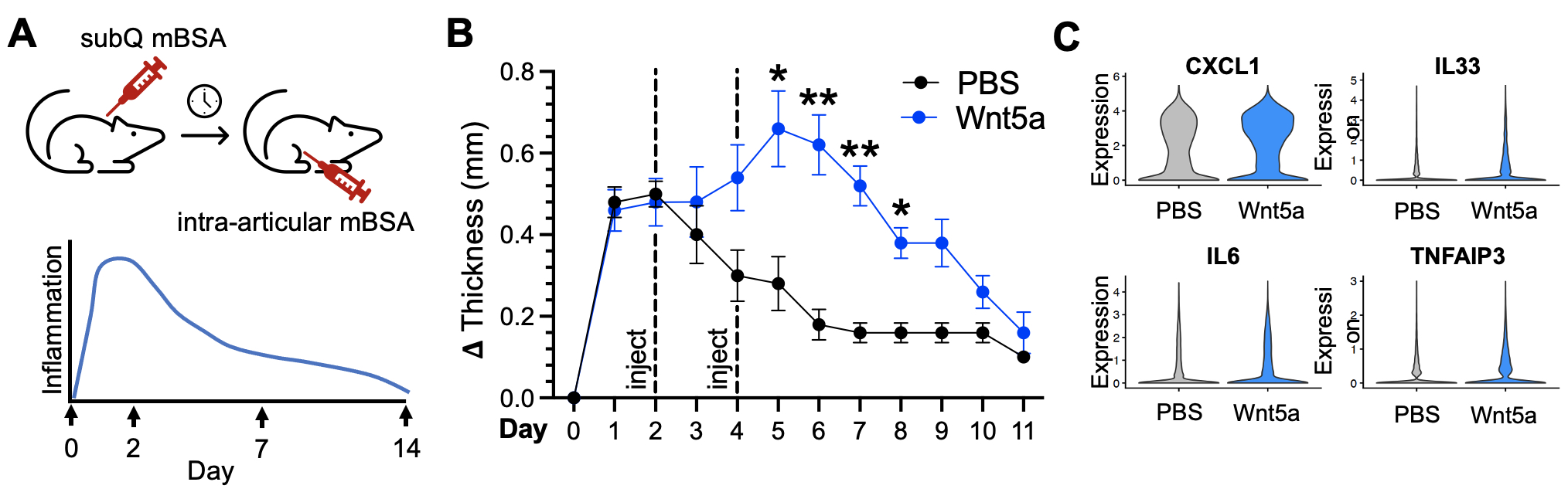
Results: Synovial tissues were processed at baseline, peak inflammation, resolving and resolved time points in K/BxN serum transfer recipient mice (Figure 1A). scRNA-seq analyses reveal reciprocal expression of RSPO3 and DKK3, similar to the pattern in human RA synovium (Figure 1B and 1C). Likewise, Wnt activation scores are enriched in cells that show high expression of the Wnt agonist RSPO3 (Figure 1D). Strikingly, RSPO3 and Wnt activation signatures are increased in synovial fibroblasts isolated at the peak of arthritis compared to those from control and resolution time points (Figure 1E). To determine how the activation of Wnt signaling would impact clinical arthritis, we used the AIA model where non-canonical Wnt5a ligand could be directly injected into the knee (Figure 2A). Strikingly, we found that Wnt activation induces a profound increase in the severity and duration of arthritis (Figure 2B) associated with increased expression of cytokines, chemokines, and other inflammatory mediators in synovial fibroblasts (Figure 2C).
Conclusion: Here, we demonstrate that stromal activation of Wnt signaling identified in human RA is recapitulated in mouse models of inflammatory arthritis and that non-canonical Wnt activation is associated with worsening severity of clinical arthritis. This work provides evidence supporting a new molecular target for treatments directed at synovial fibroblasts in RA.
A. Mueller: None; A. Zou: None; L. Marsh: None; S. Kemble: None; S. Nayar: None; E. Taylor: None; T. Major: None; D. Gardner: None; G. Watts: None; C. Murphy: None; R. Fibroblast Network Consortium: Roche, 3; A. Croft: None; A. Filer: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 5, Janssen, 5, Nascient, 5, Sonoma Biotherapeutics, 2; C. Buckley: Bristol-Myers Squibb(BMS), 5, Mestag, 11; K. Wei: 10X Genomics, 5, capital one, 6, Gilead sciences, 5, horizon therapeutics, 6, Mestag, 2; i. Korsunsky: Mestag Therapeutics, 2; S. Raychaudhuri: AbbVie, 6, Janssen, 1, Mestag, Inc, 2, 8, Pfizer, 1, Sanofi, 1, Sonoma, 1, 8; M. Brenner: 4FO Ventures, 2, GlaxoSmithKlein(GSK), 2, Mestag Therapeutics, 2, 11, Third Rock Ventures, 2.
Background/Purpose: Synovial fibroblasts are a promising therapeutic target in rheumatoid arthritis (RA) where they can adopt inflammatory or destructive phenotypes and directly promote bone and cartilage degradation. In our prior analyses, we found that non-canonical Wnt activation induces robust inflammatory gene expression in these cells. Moreover, two Wnt modulators, RSPO3 and DKK3, are reciprocally expressed and form a transcriptional gradient associated with increased and decreased Wnt activation, respectively. Strikingly, RSPO3 and Wnt activation signatures are enhanced in synovial fibroblasts in RA, suggesting the potential relevance of the Wnt pathway in vivo. Here, we explore the clinical impact of Wnt signaling in mouse models of inflammatory arthritis where we demonstrate that Wnt activation worsens the severity and prolongs the course of arthritis.
Methods: Single-cell RNA sequencing (scRNA-seq) libraries were generated from enzymatically digested, sort-purified CD45- synovial tissue dissected from K/BxN serum recipient mice at the indicated time points. To generate Wnt activation signatures, human RA synovial fibroblasts were stimulated in vitro with a combination of Wnt ligands, doses, and time points, and we performed RNA-seq and differential gene expression analysis. RSPO3 signatures were developed from scRNA-seq data of synovial fibroblasts from the Roche Fibroblast Network Consortium. In the antigen-induced arthritis (AIA) model, C57Bl/6 mice underwent subcutaneous (day -21) and knee intra-articular (day 0) injections of methylated BSA (mBSA) followed by knee intra-articular injections of PBS or Wnt5a (days 2 and 4). Knee diameters were measured using calipers, and synovial samples were collected at day 7 for scRNA-seq.
Results: Synovial tissues were processed at baseline, peak inflammation, resolving and resolved time points in K/BxN serum transfer recipient mice (Figure 1A). scRNA-seq analyses reveal reciprocal expression of RSPO3 and DKK3, similar to the pattern in human RA synovium (Figure 1B and 1C). Likewise, Wnt activation scores are enriched in cells that show high expression of the Wnt agonist RSPO3 (Figure 1D). Strikingly, RSPO3 and Wnt activation signatures are increased in synovial fibroblasts isolated at the peak of arthritis compared to those from control and resolution time points (Figure 1E). To determine how the activation of Wnt signaling would impact clinical arthritis, we used the AIA model where non-canonical Wnt5a ligand could be directly injected into the knee (Figure 2A). Strikingly, we found that Wnt activation induces a profound increase in the severity and duration of arthritis (Figure 2B) associated with increased expression of cytokines, chemokines, and other inflammatory mediators in synovial fibroblasts (Figure 2C).
Conclusion: Here, we demonstrate that stromal activation of Wnt signaling identified in human RA is recapitulated in mouse models of inflammatory arthritis and that non-canonical Wnt activation is associated with worsening severity of clinical arthritis. This work provides evidence supporting a new molecular target for treatments directed at synovial fibroblasts in RA.

Figure 1: Stromal Wnt activation observed in rheumatoid arthritis is recapitulated in a murine model of inflammatory arthritis. A) Schematic depicting the methodology and time course of inflammation in the K/BxN serum transfer mouse model of inflammatory arthritis. (B) UMAP showing synovial fibroblasts from scRNA-seq analysis of joint tissues harvested at the indicated time points from C57BL/6 mice undergoing serum transfer arthritis. N=3 mice per time point. (C) UMAP of synovial fibroblasts depicting expression of the indicated genes. Arrowheads highlight regions of high expression of RSPO3 or DKK3. (D) UMAP showing the expression of RSPO3 alongside the non-canonical Wnt5a activation score, with arrowheads highlighting regions of high expression. (E) Dot plot exhibiting the level of expression of the RSPO3 and Wnt5a activation signatures at the indicated time points.

Figure 2: Wnt pathway activation increases the severity and duration of arthritis in a murine model of inflammatory arthritis. A) Schematic illustrating the methodology and time course of the antigen-induced arthritis (AIA) mouse model. Here, mice undergo subcutaneous (subQ) mBSA injection (day -21) followed by intra-articular mBSA injection in the knee (day 0). (B) Change in knee thickness over time in mice undergoing AIA with either Wnt5a (blue) or PBS control (black) knee intra-articular injection at days 2 and 4. N=11 mice each in PBS control and Wnt5a injection groups. (C) Violin plots depicting expression of the indicated genes derived from scRNA-seq of synovial tissue harvested at day 7 from AIA mice treated with PBS or Wnt5a. N=3 mice each in PBS control and Wnt5a injection groups.
A. Mueller: None; A. Zou: None; L. Marsh: None; S. Kemble: None; S. Nayar: None; E. Taylor: None; T. Major: None; D. Gardner: None; G. Watts: None; C. Murphy: None; R. Fibroblast Network Consortium: Roche, 3; A. Croft: None; A. Filer: Bristol-Myers Squibb(BMS), 5, GlaxoSmithKlein(GSK), 5, Janssen, 5, Nascient, 5, Sonoma Biotherapeutics, 2; C. Buckley: Bristol-Myers Squibb(BMS), 5, Mestag, 11; K. Wei: 10X Genomics, 5, capital one, 6, Gilead sciences, 5, horizon therapeutics, 6, Mestag, 2; i. Korsunsky: Mestag Therapeutics, 2; S. Raychaudhuri: AbbVie, 6, Janssen, 1, Mestag, Inc, 2, 8, Pfizer, 1, Sanofi, 1, Sonoma, 1, 8; M. Brenner: 4FO Ventures, 2, GlaxoSmithKlein(GSK), 2, Mestag Therapeutics, 2, 11, Third Rock Ventures, 2.



