Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – ANCA-Associated II (2491–2496)
2491: Alignment Between the Novel 2022 ACR/EULAR Classification Criteria for ANCA-associated Vasculitis (AAV), Clinical Diagnosis and Organ Manifestations in a European AAV Cohort
Tuesday, November 14, 2023
2:00 PM - 2:10 PM PT
Location: Ballroom 20A
- TN
Thomas Neumann, MD (he/him/his)
Kantonsspital St. Gallen
Rorschacherberg, SwitzerlandDisclosure information not submitted.
Presenting Author(s)
Stefan Krämer1, Thomas Rauen1, Kristian Vogt1, Teresa Anslinger1, Martin Busch2, Tobias Schmitt3, Raoul Bergner4, Sebastian Mosberger4 and Thomas Neumann5, 1RWTH, University Hospital Aachen, Department of Nephrology and Rheumatology, Aachen, Germany, 2University Hospital Jena, Friedrich-Schiller University, Department of Internal Mediciine III, Jena, Germany, 3University Hospital Jena, Friedrich Schiller University, Department of Nephrology, Jena, Germany, 4Municipal Hospital Ludwigshafen, Department of Internal Medicine A, Nephrology and Rheumatology, Ludwigshafen, Germany, 5Cantonal Hospital St. Gallen, Department of Rheumatology, St. Gallen, Switzerland
Background/Purpose: In 2022, ACR and EULAR proposed new classification criteria for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) based on a numerical item scoring system for different manifestations in ANCA-associated vasculitis (AAV) emphasizing the role of laboratory findings. Recently, a low concordance rate of only 73% in comparison with former nomenclature and algorithm for classification was reported in a Korean cohort of 65 GPA patients [1]. We thought to (i) retrospectively re-classify patients from our multicentric AAV cohort using to the 2022 ACR/EULAR criteria for GPA and MPA using a computational algorithm that involved existing clinical, laboratory and histological data, (ii) to compare these results to prior clinical diagnoses and (iii) to investigate the predictive power of organ manifestations for both, classification and diagnosis.
Methods: Data of AAV patients from four tertiary referral centers (Germany and Switzerland) were collected between 2000 and 2021. Cases without conclusive ANCA status were excluded from computed analysis. The 2022 ACR/EULAR criteria were applied by algorithmically analyzing BVAS entries for organ manifestation, laboratory results for ANCA/ELISA testing and histological data for detection of granuloma and/or pauci-immune glomerulonephritis. Results were compared to previously reported diagnoses and clinical manifestations were analyzed by their odds ratio (OR) to be either diagnosed or classified as GPA or MPA.
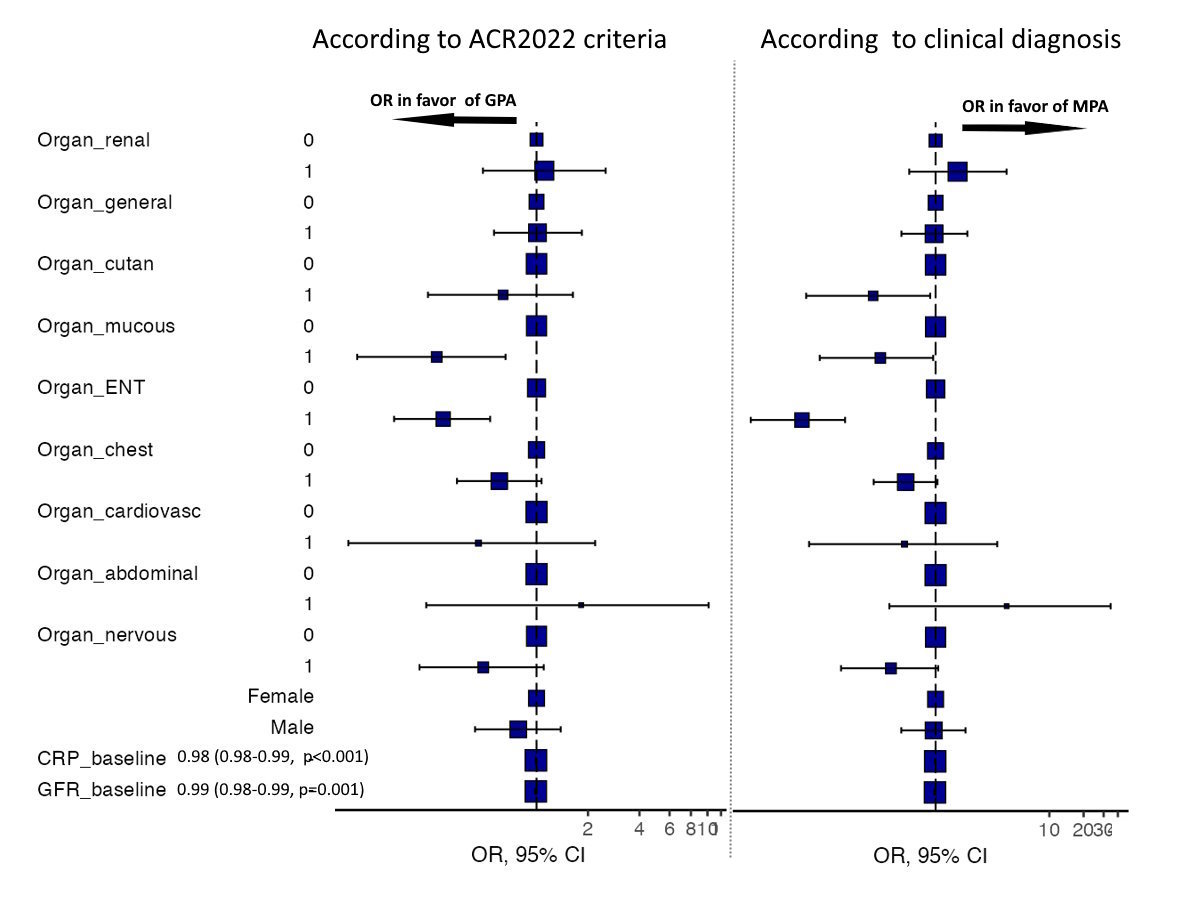
Results: The final dataset included a total of 305 cases, 294 (96.4%) of Caucasian ethnicity, 161 males (52.8%), median age 61 years (IQR 50-70). Based on the 2022 ACR/EULAR criteria, 299 (98%) cases could be unambiguously categorized. The overall concordance was found to be higher in GPA with 93.1% (vs. 88.2% in MPA) as previously reported [2]. Within all cases classified as MPA, the ACR/EULAR itemized score sums up to an average of 7.99 points (±1.42) in MPA cases vs. -0.201 points (±1.36) for GPA cases (fig. 1). Classification as GPA sums up to an average score of 5.87 points (±1.21) vs. 0.548 points (±1.68). Overall, the distance of 9.41 points is higher in MPA cases, indicating a better selectivity as compared to GPA than vice versa with 5.32 points. ENT/mucous membrane involvement showed significant association with GPA for both classification and even more in clinical diagnosis (fig. 2). In addition to BVAS, baseline CRP (100 vs. 56 mg/L) and estimated GFR levels (57 vs. 36 ml/min/1.73 m²) were more elevated in GPA. GPA was more common among males than MPA.
Conclusion: The computational algorithm performed well to categorize 98% of all cases. Our findings demonstrated positive and negative concordance rates above 88% and suggest a higher specificity for GPA, but higher sensitivity for MPA classification criteria as well as better selectivity in MPA. Concerning clinical manifestations, ENT and mucous membrane involvement showed highest and significant association to GPA in our European cohort, reflecting their strong weighting in scoring.
.jpg)

S. Krämer: None; T. Rauen: None; K. Vogt: None; T. Anslinger: None; M. Busch: AstraZeneca, 6, Boehringer-Ingelheim, 2, 6, GlaxoSmithKline(GSK), 2, 6, Novartis, 6, Pfizer, 6, Vifor, 2, 6; T. Schmitt: None; R. Bergner: AbbVie/Abbott, 6, Bristol-Myers Squibb(BMS), 6, Chugai, 6, Galapagos, 2, 6, GlaxoSmithKlein(GSK), 6, Merck/MSD, 6, Novartis, 6, Vifor, 2; S. Mosberger: None; T. Neumann: GlaxoSmithKlein(GSK), 1, 6, Janssen, 12, Travel support, Novartis, 6, Pfizer, 12, Travel support, Vifor Pharma Switzerland, 1, 5, 6.
Background/Purpose: In 2022, ACR and EULAR proposed new classification criteria for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) based on a numerical item scoring system for different manifestations in ANCA-associated vasculitis (AAV) emphasizing the role of laboratory findings. Recently, a low concordance rate of only 73% in comparison with former nomenclature and algorithm for classification was reported in a Korean cohort of 65 GPA patients [1]. We thought to (i) retrospectively re-classify patients from our multicentric AAV cohort using to the 2022 ACR/EULAR criteria for GPA and MPA using a computational algorithm that involved existing clinical, laboratory and histological data, (ii) to compare these results to prior clinical diagnoses and (iii) to investigate the predictive power of organ manifestations for both, classification and diagnosis.
Methods: Data of AAV patients from four tertiary referral centers (Germany and Switzerland) were collected between 2000 and 2021. Cases without conclusive ANCA status were excluded from computed analysis. The 2022 ACR/EULAR criteria were applied by algorithmically analyzing BVAS entries for organ manifestation, laboratory results for ANCA/ELISA testing and histological data for detection of granuloma and/or pauci-immune glomerulonephritis. Results were compared to previously reported diagnoses and clinical manifestations were analyzed by their odds ratio (OR) to be either diagnosed or classified as GPA or MPA.
Results: The final dataset included a total of 305 cases, 294 (96.4%) of Caucasian ethnicity, 161 males (52.8%), median age 61 years (IQR 50-70). Based on the 2022 ACR/EULAR criteria, 299 (98%) cases could be unambiguously categorized. The overall concordance was found to be higher in GPA with 93.1% (vs. 88.2% in MPA) as previously reported [2]. Within all cases classified as MPA, the ACR/EULAR itemized score sums up to an average of 7.99 points (±1.42) in MPA cases vs. -0.201 points (±1.36) for GPA cases (fig. 1). Classification as GPA sums up to an average score of 5.87 points (±1.21) vs. 0.548 points (±1.68). Overall, the distance of 9.41 points is higher in MPA cases, indicating a better selectivity as compared to GPA than vice versa with 5.32 points. ENT/mucous membrane involvement showed significant association with GPA for both classification and even more in clinical diagnosis (fig. 2). In addition to BVAS, baseline CRP (100 vs. 56 mg/L) and estimated GFR levels (57 vs. 36 ml/min/1.73 m²) were more elevated in GPA. GPA was more common among males than MPA.
Conclusion: The computational algorithm performed well to categorize 98% of all cases. Our findings demonstrated positive and negative concordance rates above 88% and suggest a higher specificity for GPA, but higher sensitivity for MPA classification criteria as well as better selectivity in MPA. Concerning clinical manifestations, ENT and mucous membrane involvement showed highest and significant association to GPA in our European cohort, reflecting their strong weighting in scoring.
.jpg)
Clustering of ACR/EULAR 2022 criteria scores for GPA (left) and GPA classified cases (right)

OR for either MPA or GPA classification (left) and previous clinical diagnosis (right)

Literature
S. Krämer: None; T. Rauen: None; K. Vogt: None; T. Anslinger: None; M. Busch: AstraZeneca, 6, Boehringer-Ingelheim, 2, 6, GlaxoSmithKline(GSK), 2, 6, Novartis, 6, Pfizer, 6, Vifor, 2, 6; T. Schmitt: None; R. Bergner: AbbVie/Abbott, 6, Bristol-Myers Squibb(BMS), 6, Chugai, 6, Galapagos, 2, 6, GlaxoSmithKlein(GSK), 6, Merck/MSD, 6, Novartis, 6, Vifor, 2; S. Mosberger: None; T. Neumann: GlaxoSmithKlein(GSK), 1, 6, Janssen, 12, Travel support, Novartis, 6, Pfizer, 12, Travel support, Vifor Pharma Switzerland, 1, 5, 6.



