Abstract Session
Osteoarthritis (OA) and related disorders
Session: Abstracts: Osteoarthritis & Joint Biology – Basic Science (1591–1596)
1592: Memantine Attenuates the Development of Osteoarthritis by Blocking NMDA Receptor Mediated Calcium Overload and Chondrocyte Senescence
Monday, November 13, 2023
2:15 PM - 2:25 PM PT
Location: Room 30D-E
- NW
Presenting Author(s)
Ning Wang1, Qingmei Cheng1, Xiaoxiao Li2, ke he1, Junyu Zhu1, Hui Li3 and Jie Wei4, 1中南大学湘雅医院, Changsha, China, 2Key Laboratory of Aging-related Bone and Joint Diseases Prevention and Treatment, Ministry of Education, Xiangya Hospital, Central South University, Changsha, China, 3Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China, 4Health Management Center, Xiangya Hospital Central South University, Changsha, China
Background/Purpose: Memantine is an FDA proved drug utilized for the treatment of dementia and exerts its function by blocking the NMDA (N-methyl-D-aspartate) receptor, a calcium permeable ion channel that reduces cytotoxic calcium overload. Chondrocyte senescence is a crucial cellular event that contributes to articular cartilage degeneration during osteoarthritis (OA) development. However, little information has been reported regarding the effects of memantine and its downstream NMDA receptor on chondrocyte senescence and OA.
Methods: The protein levels of the NMDA receptor and its agonistic ligand, glutamate, were compared in OA and normal chondrocytes. The amount of intracellular calcium ions and mitochondrial damage were evaluated using specific fluorescent probes and transmission electron microscopy (TEM). Senescence-associated β-galactosidase (SA-β-gal) staining and p16INK4a were analyzed to assess chondrocyte senescence. The function of the NMDA receptor in chondrocyte senescence and OA were tested through agonists activation and gene knockout experiments. The therapeutic effects of memantine on OA were tested both in vitro and in vivo. Additionally, to verify the findings from animal samples, a propensity score matched human cohort study using the IQVIA Medical Research Data primary care database in the UK was conducted to compare the risk of OA associated joint replacement (the clinically relevant endpoint of OA) in memantine initiators with initiators of active comparator, i.e., acetylcholinesterase (AchE), among patients with dementia.
Results: The protein expression of the NMDA receptor and the secretion of glutamate were both significantly higher in OA chondrocytes. Activation of the NMDA receptor stimulated chondrocyte calcium overload, which resulted in mitochondrial fragmentation and chondrocyte senescence. Inhibiting the NMDA receptor by memantine and knockout NR1, the gene encoding NMDA receptor, resulted in reduced calcium influx, mitochondrial fragmentation as well as cellular senescence in OA chondrocyte. In addition, intra-articular injection of memantine in OA mice model exhibited protective effects on cartilage degeneration. What's more, in the 1:5 propensity-score matched cohort study consisted of 6,218 patients (n=1,435 in memantine cohort; n=4,783 in AchE cohort), memantine initiators was associated with a lower risk of OA-associated joint replacement compared with AchE initiators (hazard ratio=0.57, 95% confidence interval: 0.34 to 0.99).
Conclusion: As a clinically licensed drug used for dementia, memantine shows promising therapeutic effects on OA. Mechanistically, it blocks NMDA receptor-mediated chondrocyte senescence. The protective effects of memantine on OA were verified not only through in vitro and in vivo experiments but also via a propensity-score matched human cohort study. These findings present robust evidence for repurposing memantine for the treatment of OA.
N. Wang: None; Q. Cheng: None; X. Li: None; k. he: None; J. Zhu: None; H. Li: None; J. Wei: None.
Background/Purpose: Memantine is an FDA proved drug utilized for the treatment of dementia and exerts its function by blocking the NMDA (N-methyl-D-aspartate) receptor, a calcium permeable ion channel that reduces cytotoxic calcium overload. Chondrocyte senescence is a crucial cellular event that contributes to articular cartilage degeneration during osteoarthritis (OA) development. However, little information has been reported regarding the effects of memantine and its downstream NMDA receptor on chondrocyte senescence and OA.
Methods: The protein levels of the NMDA receptor and its agonistic ligand, glutamate, were compared in OA and normal chondrocytes. The amount of intracellular calcium ions and mitochondrial damage were evaluated using specific fluorescent probes and transmission electron microscopy (TEM). Senescence-associated β-galactosidase (SA-β-gal) staining and p16INK4a were analyzed to assess chondrocyte senescence. The function of the NMDA receptor in chondrocyte senescence and OA were tested through agonists activation and gene knockout experiments. The therapeutic effects of memantine on OA were tested both in vitro and in vivo. Additionally, to verify the findings from animal samples, a propensity score matched human cohort study using the IQVIA Medical Research Data primary care database in the UK was conducted to compare the risk of OA associated joint replacement (the clinically relevant endpoint of OA) in memantine initiators with initiators of active comparator, i.e., acetylcholinesterase (AchE), among patients with dementia.
Results: The protein expression of the NMDA receptor and the secretion of glutamate were both significantly higher in OA chondrocytes. Activation of the NMDA receptor stimulated chondrocyte calcium overload, which resulted in mitochondrial fragmentation and chondrocyte senescence. Inhibiting the NMDA receptor by memantine and knockout NR1, the gene encoding NMDA receptor, resulted in reduced calcium influx, mitochondrial fragmentation as well as cellular senescence in OA chondrocyte. In addition, intra-articular injection of memantine in OA mice model exhibited protective effects on cartilage degeneration. What's more, in the 1:5 propensity-score matched cohort study consisted of 6,218 patients (n=1,435 in memantine cohort; n=4,783 in AchE cohort), memantine initiators was associated with a lower risk of OA-associated joint replacement compared with AchE initiators (hazard ratio=0.57, 95% confidence interval: 0.34 to 0.99).
Conclusion: As a clinically licensed drug used for dementia, memantine shows promising therapeutic effects on OA. Mechanistically, it blocks NMDA receptor-mediated chondrocyte senescence. The protective effects of memantine on OA were verified not only through in vitro and in vivo experiments but also via a propensity-score matched human cohort study. These findings present robust evidence for repurposing memantine for the treatment of OA.

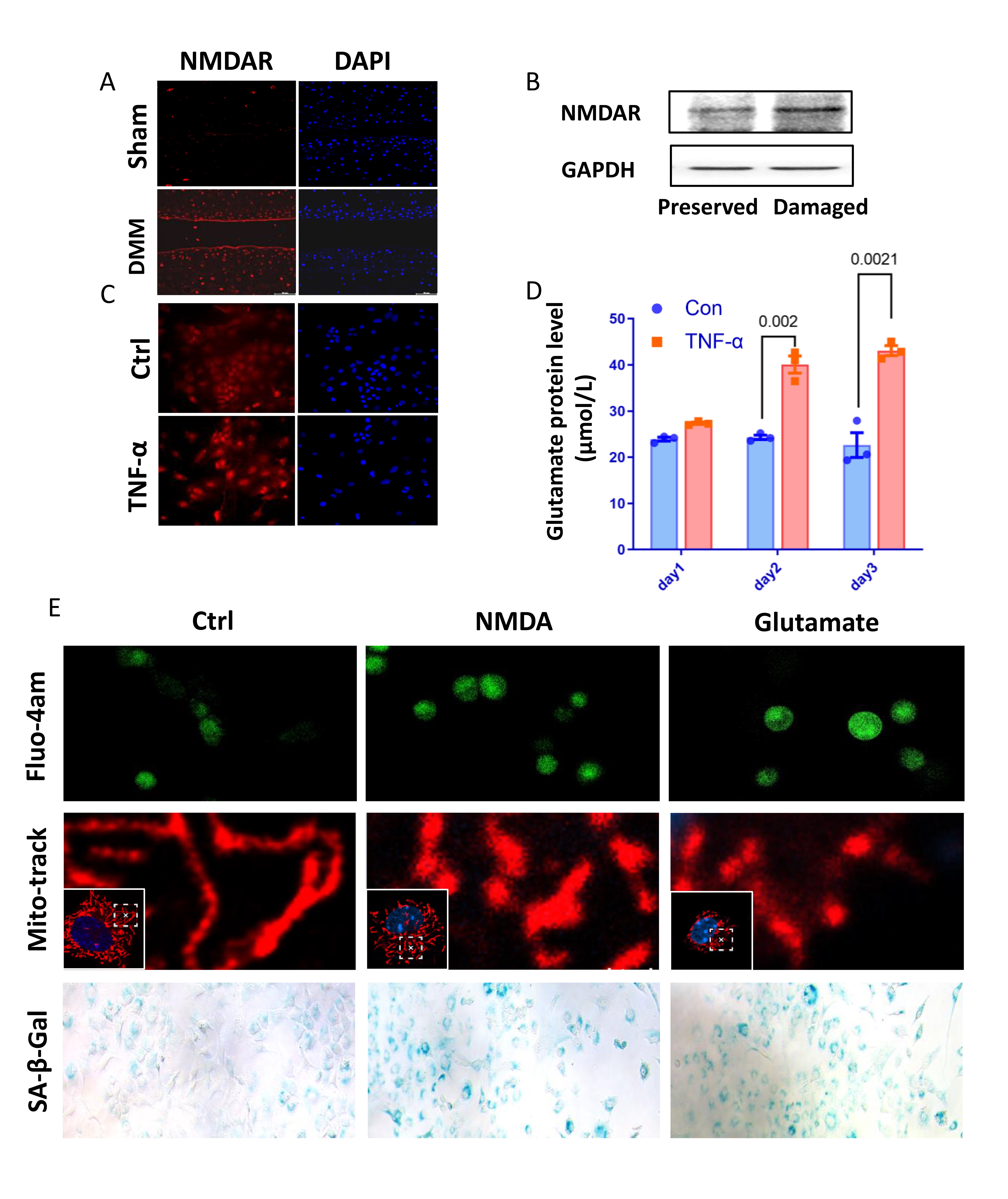
Figure 1. OA progression accompanied by highly expressed and activated levels of NMDA receptor that led to mitochondrial fragmentation and chondrocyte senescence. Immunostaining was used to analyze the level of NMDA receptor in the destabilization of the medial meniscus (DMM) surgery-induced OA mice knee joint (A), as well as the inflammatory-induced OA chondrocyte (C). The protein level of NMDA receptor in preserved and damaged cartilage from OA patients (B). The secretion of glutamate from OA chondrocytes in cell culture medium (D). The calcium probe Fluo-4am, the mitochondrial probe mito-tracker, and the SA-β-gal staining were used for chondrocytes stimulated with NMDA or glutamate (E).

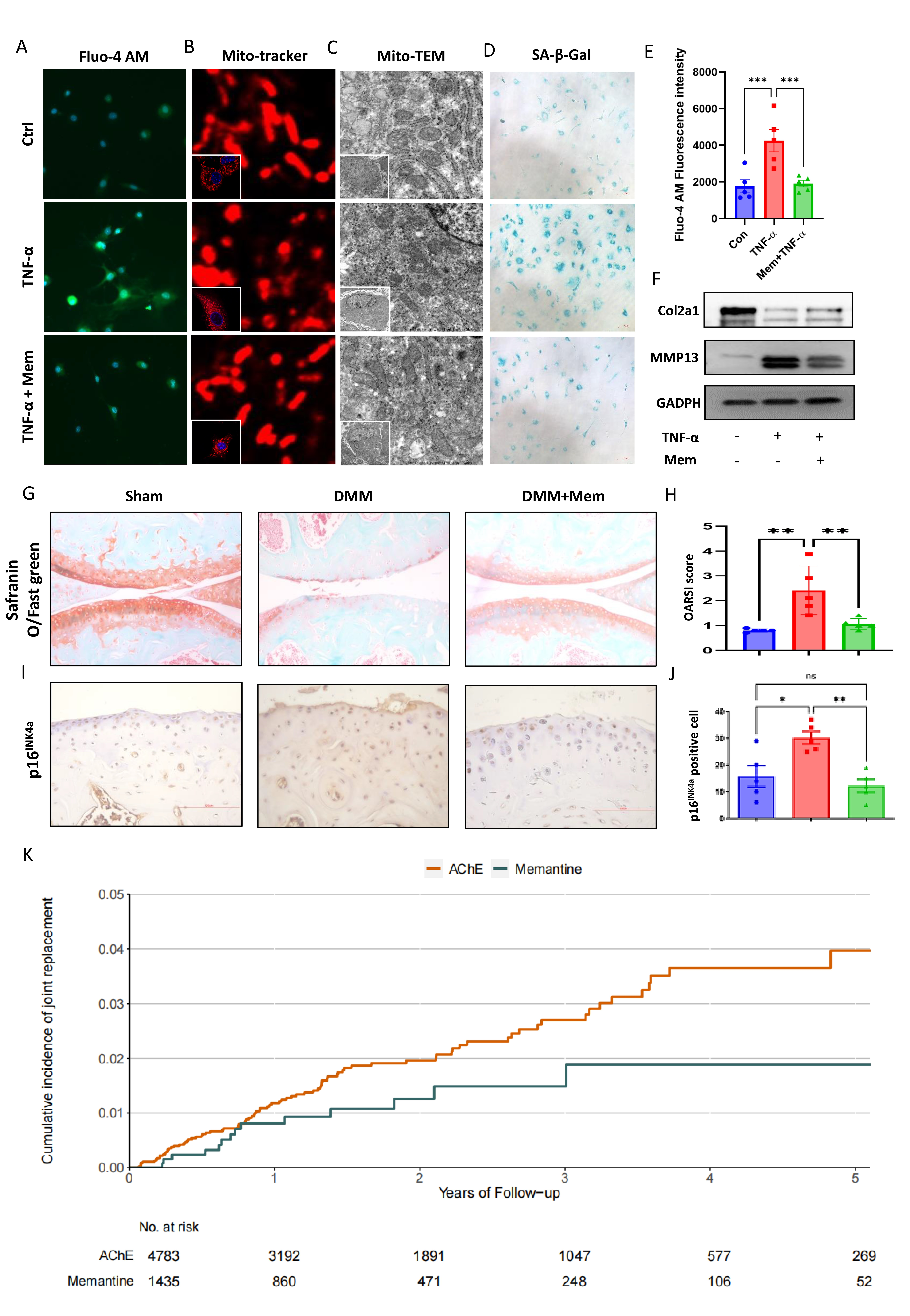
Figure 2. Memantine protects against OA and is associated with reduced risk of knee or hip OA-associated joint replacement among patients with dementia. The Fluo-4am (A), mito-tracker (B), TEM (C), and SA-β-gal staining (D) were used for OA chondrocytes stimulated with Memantine or not. Semi-quantification of calcium overload based on the staining (E). Protein levels of Col2a1 and MMP13 were detected (F). Safranin O/Fast green staining (G) and OARSI score (H), p16INK4a immunohistochemistry staining (I), and semi-quantification (J) for DMM-induced OA model. Cumulative incidence of knee or hip osteoarthritis-associated joint replacement in 1,435 memantine users and 4,783 AchE users (K), matched by propensity score.

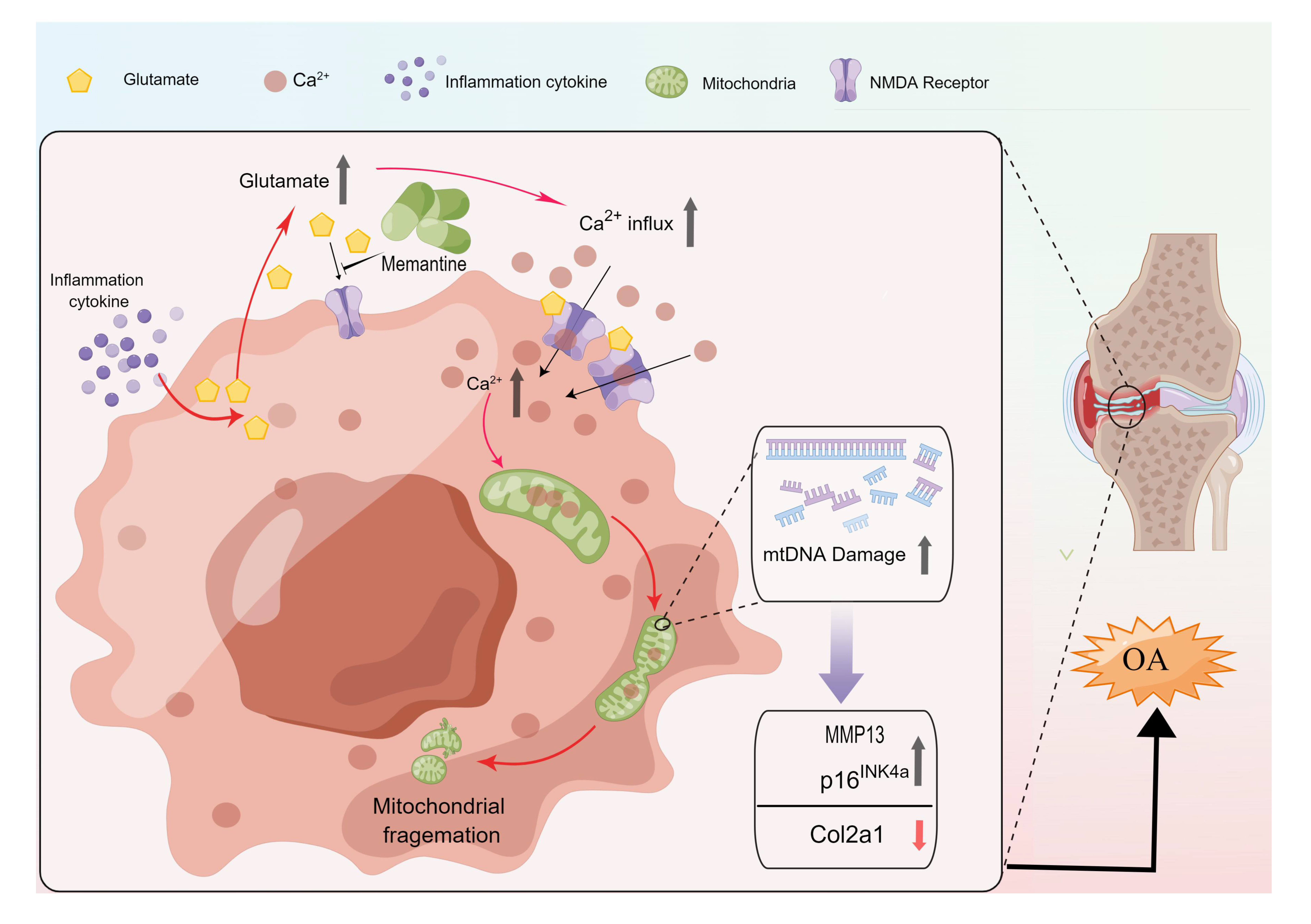
Figure 3. The mechanistic diagram of memantine as a potential chondroprotective drug for OA.
N. Wang: None; Q. Cheng: None; X. Li: None; k. he: None; J. Zhu: None; H. Li: None; J. Wei: None.



