Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Health Services Research II (1615–1620)
1615: Therapeutic Hydroxychloroquine Blood Levels Are Cost Effective and May Reduce Health Disparities by Reducing Lupus Hospitalizations
Monday, November 13, 2023
2:00 PM - 2:10 PM PT
Location: Room 33A-C
- SG
Shivani Garg, MD, MS
University of Madison, School of Medicine and Public Health
Madison, WI, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Presenting Author(s)
Shivani Garg1, Giancarlo Valiente2, Lexie Kolton3, Callie Saric3, Betty Chewning4 and Christie M. Bartels2, 1Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, 2University of Wisconsin, School of Medicine and Public Health, Madison, WI, 3University of Wisconsin, Madison, WI, 4University of Wisconsin, School of Pharmacy, Madison, WI
Background/Purpose: Studies show factors including daily hydroxychloroquine (HCQ) dosing or nonadherence affect blood concentrations risking 6-fold higher lupus (or SLE) flares requiring hospitalization. Given disparities in nonadherence resulting in more hospitalizations, measuring HCQ levels in blood could guide clinicians to tailor HCQ dosing to maximize efficacy and improve adherence at the individual patient level. Yet, HCQ blood level monitoring is not routinely done due to cost, coverage, and unclear clinical standardization. Thus, we aimed to examine the cost effectiveness of HCQ blood level monitoring by measuring: 1) risk of lupus-related acute care utilization (Hospital or Emergency Room (ER) visits) by HCQ blood levels overall and in patients of Black race or Hispanic ethnicity; 2) the cost of lupus-related acute care visits vs. HCQ blood level monitoring.
Methods: We measured HCQ blood levels during 167 consecutive visits of unique patients using liquid chromatography mass spectrometry. HCQ blood levels were categorized as: a) subtherapeutic (< 750 ng/ml), b) therapeutic (750-1100 ng/ml), or c) supratherapeutic ( >1100 ng/ml) per our prior findings. All lupus-related acute care (hospital/ER) visits from the clinic visit until next follow-up visit were manually abstracted. Covariates included socio-demographics, SLE disease activity score, SLE damage index, kidney function (eGFR), steroid and HCQ doses. Using Cox hazard models, we examined associations between HCQ blood levels and time to first acute care visit after the index clinic visit. We compared published data (Kan, 2013) on the cost of lupus-related acute care visits to HCQ blood level monitoring in our cohort.
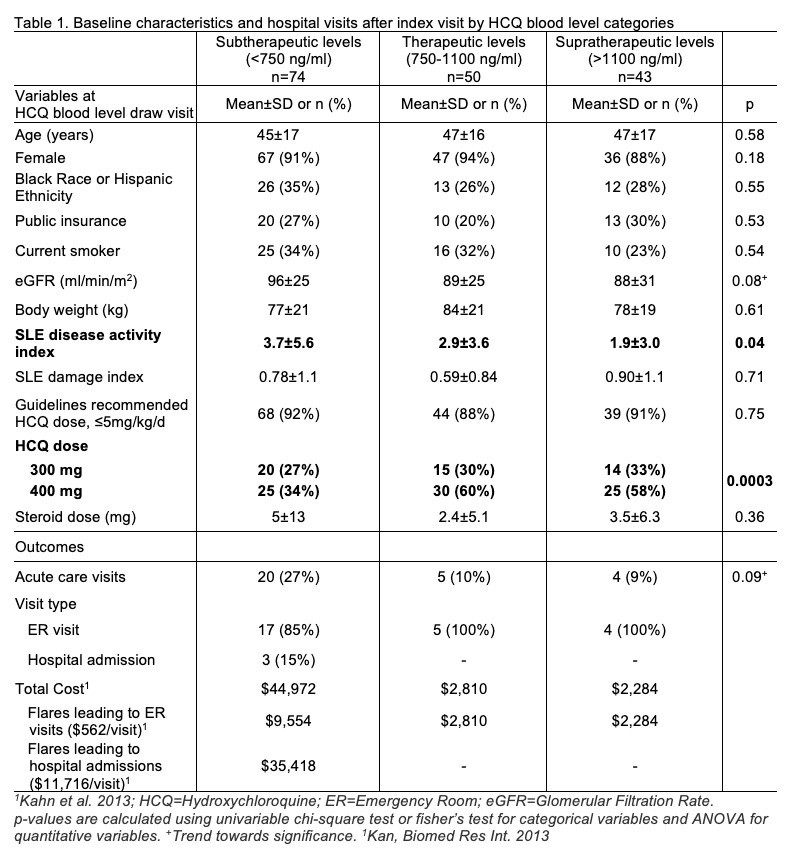
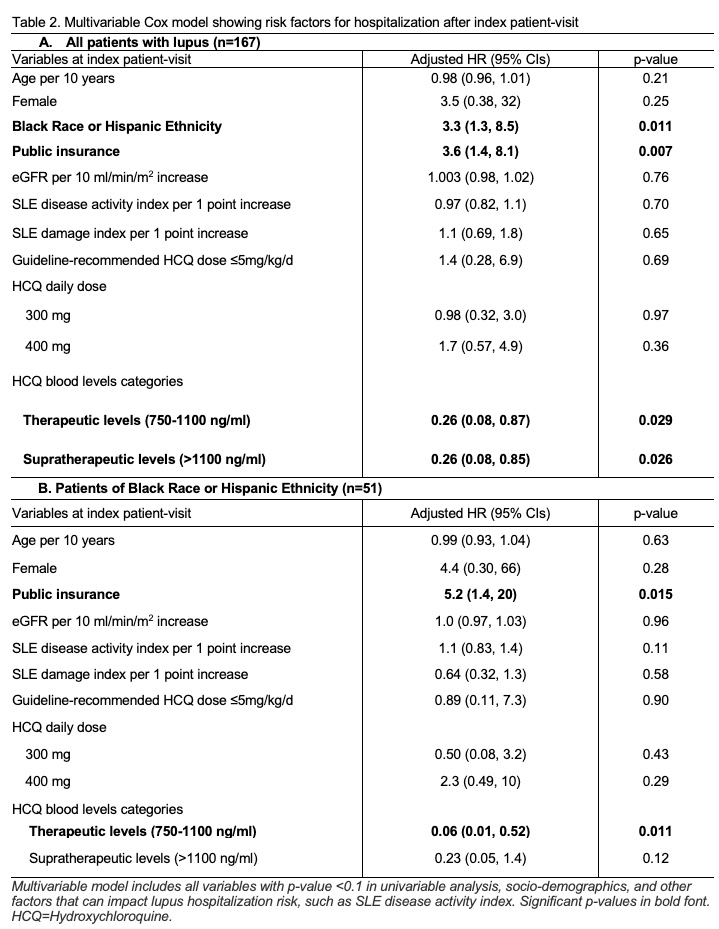
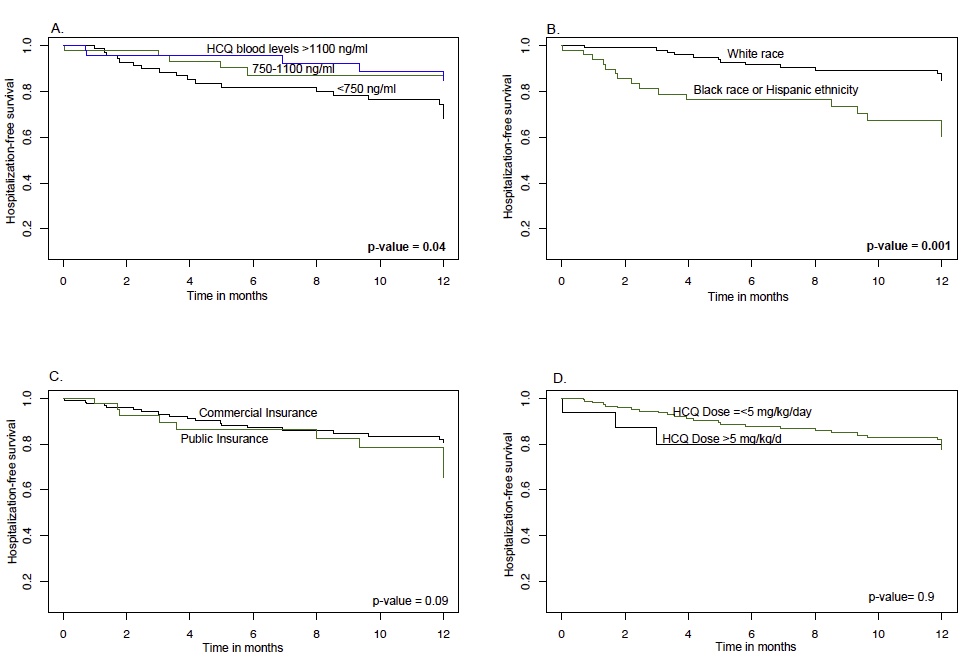
Results: Table 1 shows characteristics of 167 patients included in this study by: subtherapeutic (n=74), therapeutic (n=50), supratherapeutic (n=43) HCQ blood levels. A total of 31 lupus-related acute care visits were observed during the study period. Patients of Black race or Hispanic ethnicity and those with public insurance had 3-fold higher acute care utilization risk (Adjusted HR 3.3 & HR 3.6; Fig. 1 & Table 2A). A 71% lower acute care utilization risk was seen in patients with therapeutic HCQ blood levels, 750-1100 ng/ml, compared to those with subtherapeutic levels, < 750 ng/ml (Fig. 1 & Table 2A; p=0.029).
In a subgroup analysis among patients of Black race or Hispanic ethnicity (n=51), we noted that public insurance predicted 5-fold higher acute care utilization risk (Table 2B). Therapeutic HCQ blood levels were associated with 94% lower acute care utilization (Table 2B).
Using published costs of $562 per SLE ER visit and $11,716 per SLE hospitalization, 28 ER visits and 3 hospitalizations cost approximately $50,584 in our study. At a cost of $50 per HCQ blood level measurement, monitoring HCQ blood levels for 167 draws costs $8,350. Thereby, preventing one ER visit could cover the cost of up to 11 HCQ blood level draws per year.
Conclusion: Therapeutic HCQ blood levels (750-1100 ng/ml) were associated with 94% lower acute care utilization risk among patients of Black race or Hispanic ethnicity. Routine HCQ blood level monitoring could cost effectively reduce acute care utilization and health disparities in lupus.
S. Garg: None; G. Valiente: None; L. Kolton: None; C. Saric: None; B. Chewning: None; C. Bartels: Pfizer, 5.
Background/Purpose: Studies show factors including daily hydroxychloroquine (HCQ) dosing or nonadherence affect blood concentrations risking 6-fold higher lupus (or SLE) flares requiring hospitalization. Given disparities in nonadherence resulting in more hospitalizations, measuring HCQ levels in blood could guide clinicians to tailor HCQ dosing to maximize efficacy and improve adherence at the individual patient level. Yet, HCQ blood level monitoring is not routinely done due to cost, coverage, and unclear clinical standardization. Thus, we aimed to examine the cost effectiveness of HCQ blood level monitoring by measuring: 1) risk of lupus-related acute care utilization (Hospital or Emergency Room (ER) visits) by HCQ blood levels overall and in patients of Black race or Hispanic ethnicity; 2) the cost of lupus-related acute care visits vs. HCQ blood level monitoring.
Methods: We measured HCQ blood levels during 167 consecutive visits of unique patients using liquid chromatography mass spectrometry. HCQ blood levels were categorized as: a) subtherapeutic (< 750 ng/ml), b) therapeutic (750-1100 ng/ml), or c) supratherapeutic ( >1100 ng/ml) per our prior findings. All lupus-related acute care (hospital/ER) visits from the clinic visit until next follow-up visit were manually abstracted. Covariates included socio-demographics, SLE disease activity score, SLE damage index, kidney function (eGFR), steroid and HCQ doses. Using Cox hazard models, we examined associations between HCQ blood levels and time to first acute care visit after the index clinic visit. We compared published data (Kan, 2013) on the cost of lupus-related acute care visits to HCQ blood level monitoring in our cohort.
Results: Table 1 shows characteristics of 167 patients included in this study by: subtherapeutic (n=74), therapeutic (n=50), supratherapeutic (n=43) HCQ blood levels. A total of 31 lupus-related acute care visits were observed during the study period. Patients of Black race or Hispanic ethnicity and those with public insurance had 3-fold higher acute care utilization risk (Adjusted HR 3.3 & HR 3.6; Fig. 1 & Table 2A). A 71% lower acute care utilization risk was seen in patients with therapeutic HCQ blood levels, 750-1100 ng/ml, compared to those with subtherapeutic levels, < 750 ng/ml (Fig. 1 & Table 2A; p=0.029).
In a subgroup analysis among patients of Black race or Hispanic ethnicity (n=51), we noted that public insurance predicted 5-fold higher acute care utilization risk (Table 2B). Therapeutic HCQ blood levels were associated with 94% lower acute care utilization (Table 2B).
Using published costs of $562 per SLE ER visit and $11,716 per SLE hospitalization, 28 ER visits and 3 hospitalizations cost approximately $50,584 in our study. At a cost of $50 per HCQ blood level measurement, monitoring HCQ blood levels for 167 draws costs $8,350. Thereby, preventing one ER visit could cover the cost of up to 11 HCQ blood level draws per year.
Conclusion: Therapeutic HCQ blood levels (750-1100 ng/ml) were associated with 94% lower acute care utilization risk among patients of Black race or Hispanic ethnicity. Routine HCQ blood level monitoring could cost effectively reduce acute care utilization and health disparities in lupus.

Table 1. Baseline characteristics and hospital visits after index visit by HCQ blood level categories

Table 2. Multivariable Cox model showing risk factors for hospitalization after index patient-visit

Fig 1. Kaplan Meier curves showing hospitalization-free survival by: A. HCQ blood level categories; B. Insurance; C. Race; D. HCQ dose
S. Garg: None; G. Valiente: None; L. Kolton: None; C. Saric: None; B. Chewning: None; C. Bartels: Pfizer, 5.



