Abstract Session
Vasculitis
Session: Abstracts: Vasculitis – ANCA-Associated I (0853–0858)
0858: Randomized, Controlled, Double-Blind Trial on the Impact of Rosuvastatin on Sublinical Markers of Atherosclerosis in Patients with ANCA-Associated Vasculitis
Sunday, November 12, 2023
5:15 PM - 5:25 PM PT
Location: Ballroom 20D
- BT
Presenting Author(s)
Benjamin Terrier1, Gregory Pugnet2, Marie-Emmanuelle Sirieix3, Thomas Quemeneur4, Xavier Puéchal5, Francois Maurier6, Antoine Néel7, YGAL BENHAMOU8, Bernard Bonnotte9, Jean Schmidt10, Adrien Michon3, Antoine Baudet11, Pierre Charles12, Fleur Cohen13, Claire De Moreuil14, Emanuelle Dernis15, Pauline Belenotti16, Marc Ruivard17, Olivier Aumaitre18, Olivier Fain19, Raphaele Seror20, Cécile-Audrey Durel21, Noémie Jourde-Chiche22, Estibaliz Lazaro23, Gilles Chironi24, Guillaume Armengol25, Jeremy Bellien25, Philippe Ravaud26, Gabriel Baron26 and Loic Guillevin27, 1Department of Internal Medicine, Hôpital Cochin, AP-HP, Paris, France, 2CHU Toulouse Rangueil Service de Medecine Interne et Immunologie Clinique, Toulouse, France, 3HEGP, Paris, France, 4CH Valenciennes, Valenciennes, France, 5National Referral Center for Rare Systemic Autoimmune Diseases, Paris, France, 6Hôpitaux privés de Metz, Vaux / Frankreich, France, 7CHU Nantes, Nantes, France, 8rouen university hospital, Rouen, France, 9Department of Internal Medicine and Clinical Immunology, Dijon University Hospital, Dijon, France, 10CHU Amiens, Amiens, France, 11CH Annecy, Annecy, France, 12Institut Mutualiste Montsouris, Service de Médecine Interne, Paris, France, 13Sorbonne Université, Paris, France, 14CHU de Brest, Brest, France, 15CH Le Mans, Le Mans, France, 16CHU Marseille, Marseille, France, 17CHU Clermont Ferrand, Clermont Ferrand, France, 18CHU Saint Antoine, Paris, France, 19Hopital SAINT ANTOINE APHP, Paris, France, 20University Hospital Paris Saclay, Le Kremlin-Bicêtre, France, 21CHU Lyon, Lyon, France, 22AP-HM, Marseille, France, 23Bordeaux Hospital University, Pessac, France, 24Monaco, Monaco, Monaco, 25CHU Rouen, Rouen, France, 26Paris Cité University, Paris, France, 27University Paris Descartes, Paris, France
Background/Purpose: Despite more effective therapeutic strategies in ANCA-associated vasculitis (AAV), there is still a significant risk of morbidity and mortality, mainly due to infection, and cardiovascular disease. Carotid intima-media thickness (cIMT) is a marker of subclinical atherosclerosis associated with cardiovascular risk factors and is predictive of major cardiovascular events (MACE). We hypothesized that patients with AAV might benefit from statin treatment in primary prevention to reduce subclinical markers of atherosclerosis and the incidence of major cardiovascular events.
Methods: This phase 3, multicentre, randomized, controlled, double-blind, superiority study compared rosuvastatin with placebo in reducing the progression of subclinical markers of atherosclerosis. Patients with AAV in remission after a first flare or relapse were randomized 1:1 to receive the experimental strategy based on the use of rosuvastatin 20 mg/day or placebo for 24 months. The primary endpoint was the mean change in mean cIMT (distal wall of primary carotid arteries) at 24 months.
Results: A total of 111 participants underwent randomization (55% male, mean age 54.8 (13.3) years, 63.1% GPA, 28.8% EGPA, 8.1% MPA), with 54 participants assigned to receive rosuvastatin and 57 to placebo.
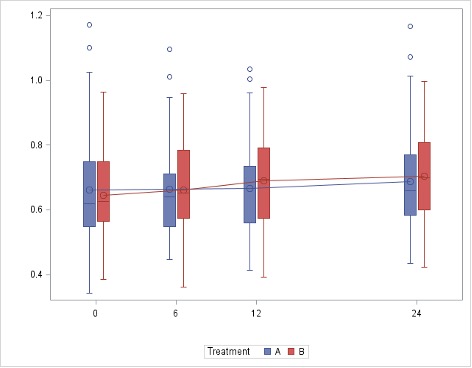
The primary endpoint was not met. The mean change in cIMT at month 24 was not different between the two study groups (difference -0.002 [-0.034 ; 0.030], p=0.89) (Figure 1). The annualized rate of change in mean cIMT was 0.0110 (0.0617) mm/year in the rosuvastatin group and 0.0189 (0.0556) mm/year in the placebo group (difference -0.0062 [-0.0318 ; 0.0193], p=0.61). Similar results were found for the mean change in the number of plaques in the carotid and femoral arteries and abdominal aorta (difference 0.01 [-0.39 ; 0.42], p=0.94).
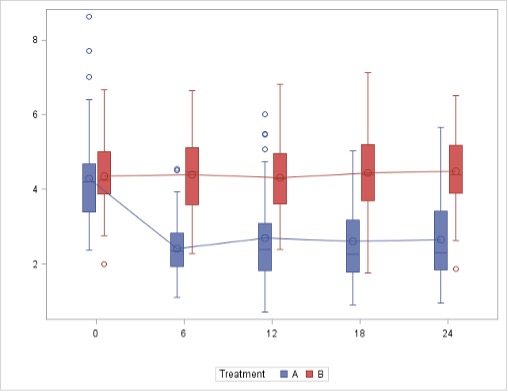
Mean LDL-cholesterol levels were significantly different between the two study groups at all time points evaluated (P< 0.001, P< 0.001, and P< 0.001 for reductions between the rosuvastatin and the placebo groups at months 6, 12 and 24, respectively) (Figure 2). Also, high-sensitivity CRP levels were significantly different between the two study groups at month 24 (difference -3.16 [-5.58 ; 0.74], p=0.011 for reductions between the rosuvastatin and the placebo groups).
There was only one MACE in the rosuvastatin group. Vasculitis relapse-free survival did not differ between the two groups (HR 1.59, 95%IC = [0.81 ; 3.09], p=0.18).
Eleven and seventeen patients discontinued intervention in the rosuvastatin and the placebo groups, respectively. The incidence of serious adverse events was similar in the two groups: 27.8% in the rosuvastatin group and 22.8% in the placebo group.
Conclusion: Among patients with ANCA-associated vasculitis, 24 months of rosuvastatin reduced LDL-cholesterol but did not reduce the progression of subclinical markers of atherosclerosis (Funded by the French Ministry of Health; STATVAS ClinicalTrials.gov number, NCT02117453).
B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2; G. Pugnet: None; M. Sirieix: None; T. Quemeneur: None; X. Puéchal: None; F. Maurier: None; A. Néel: None; Y. BENHAMOU: None; B. Bonnotte: None; J. Schmidt: None; A. Michon: None; A. Baudet: None; P. Charles: None; F. Cohen: None; C. De Moreuil: None; E. Dernis: AbbVie/Abbott, 2, Amgen, 2, Bristol-Myers Squibb(BMS), 2, Celgene, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, Janssen, 2, MSD, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Roche, 2, Roche-Chugai, 2, Sandoz, 2, Sanofi, 2, UCB Pharma, 2; P. Belenotti: None; M. Ruivard: None; O. Aumaitre: None; O. Fain: None; R. Seror: None; C. Durel: None; N. Jourde-Chiche: None; E. Lazaro: None; G. Chironi: None; G. Armengol: None; J. Bellien: None; P. Ravaud: None; G. Baron: None; L. Guillevin: None.
Background/Purpose: Despite more effective therapeutic strategies in ANCA-associated vasculitis (AAV), there is still a significant risk of morbidity and mortality, mainly due to infection, and cardiovascular disease. Carotid intima-media thickness (cIMT) is a marker of subclinical atherosclerosis associated with cardiovascular risk factors and is predictive of major cardiovascular events (MACE). We hypothesized that patients with AAV might benefit from statin treatment in primary prevention to reduce subclinical markers of atherosclerosis and the incidence of major cardiovascular events.
Methods: This phase 3, multicentre, randomized, controlled, double-blind, superiority study compared rosuvastatin with placebo in reducing the progression of subclinical markers of atherosclerosis. Patients with AAV in remission after a first flare or relapse were randomized 1:1 to receive the experimental strategy based on the use of rosuvastatin 20 mg/day or placebo for 24 months. The primary endpoint was the mean change in mean cIMT (distal wall of primary carotid arteries) at 24 months.
Results: A total of 111 participants underwent randomization (55% male, mean age 54.8 (13.3) years, 63.1% GPA, 28.8% EGPA, 8.1% MPA), with 54 participants assigned to receive rosuvastatin and 57 to placebo.
The primary endpoint was not met. The mean change in cIMT at month 24 was not different between the two study groups (difference -0.002 [-0.034 ; 0.030], p=0.89) (Figure 1). The annualized rate of change in mean cIMT was 0.0110 (0.0617) mm/year in the rosuvastatin group and 0.0189 (0.0556) mm/year in the placebo group (difference -0.0062 [-0.0318 ; 0.0193], p=0.61). Similar results were found for the mean change in the number of plaques in the carotid and femoral arteries and abdominal aorta (difference 0.01 [-0.39 ; 0.42], p=0.94).
Mean LDL-cholesterol levels were significantly different between the two study groups at all time points evaluated (P< 0.001, P< 0.001, and P< 0.001 for reductions between the rosuvastatin and the placebo groups at months 6, 12 and 24, respectively) (Figure 2). Also, high-sensitivity CRP levels were significantly different between the two study groups at month 24 (difference -3.16 [-5.58 ; 0.74], p=0.011 for reductions between the rosuvastatin and the placebo groups).
There was only one MACE in the rosuvastatin group. Vasculitis relapse-free survival did not differ between the two groups (HR 1.59, 95%IC = [0.81 ; 3.09], p=0.18).
Eleven and seventeen patients discontinued intervention in the rosuvastatin and the placebo groups, respectively. The incidence of serious adverse events was similar in the two groups: 27.8% in the rosuvastatin group and 22.8% in the placebo group.
Conclusion: Among patients with ANCA-associated vasculitis, 24 months of rosuvastatin reduced LDL-cholesterol but did not reduce the progression of subclinical markers of atherosclerosis (Funded by the French Ministry of Health; STATVAS ClinicalTrials.gov number, NCT02117453).

Figure 1. Evolution of mean carotid intima-media thickness (in mm) during the study period in the two groups. Rosuvastatin arm is group A and placebo arm is group B.

Figure 2. Evolution of mean LDL-cholesterol (in mmol/L) during the study period in the two groups. Rosuvastatin arm is group A and placebo arm is group B.
B. Terrier: AstraZeneca, 5, CSL Vifor, 2, GlaxoSmithKlein(GSK), 2; G. Pugnet: None; M. Sirieix: None; T. Quemeneur: None; X. Puéchal: None; F. Maurier: None; A. Néel: None; Y. BENHAMOU: None; B. Bonnotte: None; J. Schmidt: None; A. Michon: None; A. Baudet: None; P. Charles: None; F. Cohen: None; C. De Moreuil: None; E. Dernis: AbbVie/Abbott, 2, Amgen, 2, Bristol-Myers Squibb(BMS), 2, Celgene, 2, Eli Lilly, 2, Galapagos, 2, Gilead, 2, Janssen, 2, MSD, 2, Nordic Pharma, 2, Novartis, 2, Pfizer, 2, Roche, 2, Roche-Chugai, 2, Sandoz, 2, Sanofi, 2, UCB Pharma, 2; P. Belenotti: None; M. Ruivard: None; O. Aumaitre: None; O. Fain: None; R. Seror: None; C. Durel: None; N. Jourde-Chiche: None; E. Lazaro: None; G. Chironi: None; G. Armengol: None; J. Bellien: None; P. Ravaud: None; G. Baron: None; L. Guillevin: None.



