Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Biomarkers (0847–0852)
0847: Development of an IFN 5-Gene Signature Score to Identify IFN-high and IFN-low Subsets and as a Pharmacodynamic Biomarker for Deucravacitinib Treatment in a Phase 2 Trial in Patients with Systemic Lupus Erythematosus
Sunday, November 12, 2023
4:00 PM - 4:10 PM PT
Location: Exhibit Hall A-B
- IC
Ian Catlett, PhD
Bristol Meyers Squibb
Pennington, NJ, United StatesDisclosure information not submitted.
Presenting Author(s)
Chun Wu1, Yanhua Hu1, Mary K. Crow2, Amit Saxena3, Cristina Arriens4, Coburn Hobar1, Adrian Coles5 and Ian M. Catlett6, 1Bristol Myers Squibb, Princeton, NJ, 2Hospital for Special Surgery, New York, NY, 3NYU Langone, New York, NY, 4Oklahoma Medical Research Foundation and University of Oklahoma Health Sciences Center, Department of Arthritis & Clinical Immunology, Oklahoma City, OK, 5Bristol Myers Squibb, Lawrenceville, NJ, 6Bristol Myers Squibb, Pennington, NJ
Background/Purpose: Elevated IFN activity is observed in a subset of lupus subjects suggesting that those with higher levels of IRGs may benefit from interferon targeted therapies, however, to date responses by this dichotomization have been inconsistent. Tyrosine kinase 2 (TYK2) mediates cytokine pathways (eg, type I IFN, IL-12 and IL-23) linked with SLE pathogenesis. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor and was found to be efficacious in a phase 2 SLE trial.1 We developed a customized IFN 5-gene signature score, assessed the pharmacodynamic effects of deucravacitinib on the IFN score, and evaluated the score's association with SLE disease activity and clinical response in the phase 2 trial (NCT03252587).
Methods: Patients were randomized equally to placebo or deucravacitinib (3 mg twice daily [BID], 6 mg BID, or 12 mg once daily [QD]). DxTerity chemical ligation-dependent probe amplification was used to measure 51 immune system–related genes from whole blood. IFN genes were selected based on distribution, correlations, hierarchical clustering, and consistency of k-means clusters. Serum proteins, blood cell subsets, and antibodies were measured by immunoassays and flow cytometry. Systemic Lupus Erythematosus Responder Index-4 (SRI[4]) and British Isles Assessment Group–based Composite Lupus Assessment (BICLA) were measured at weeks 32 and 48.
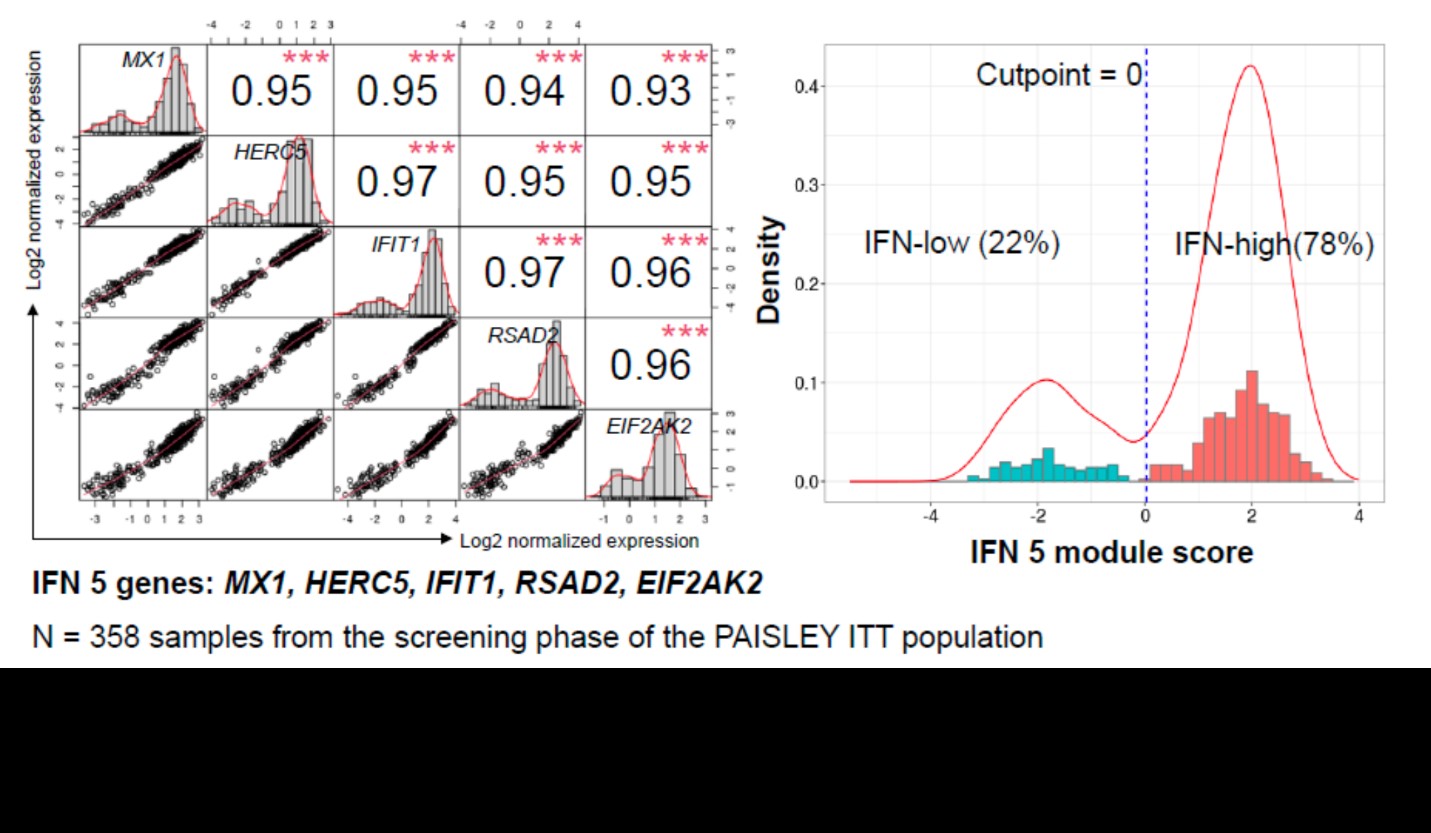
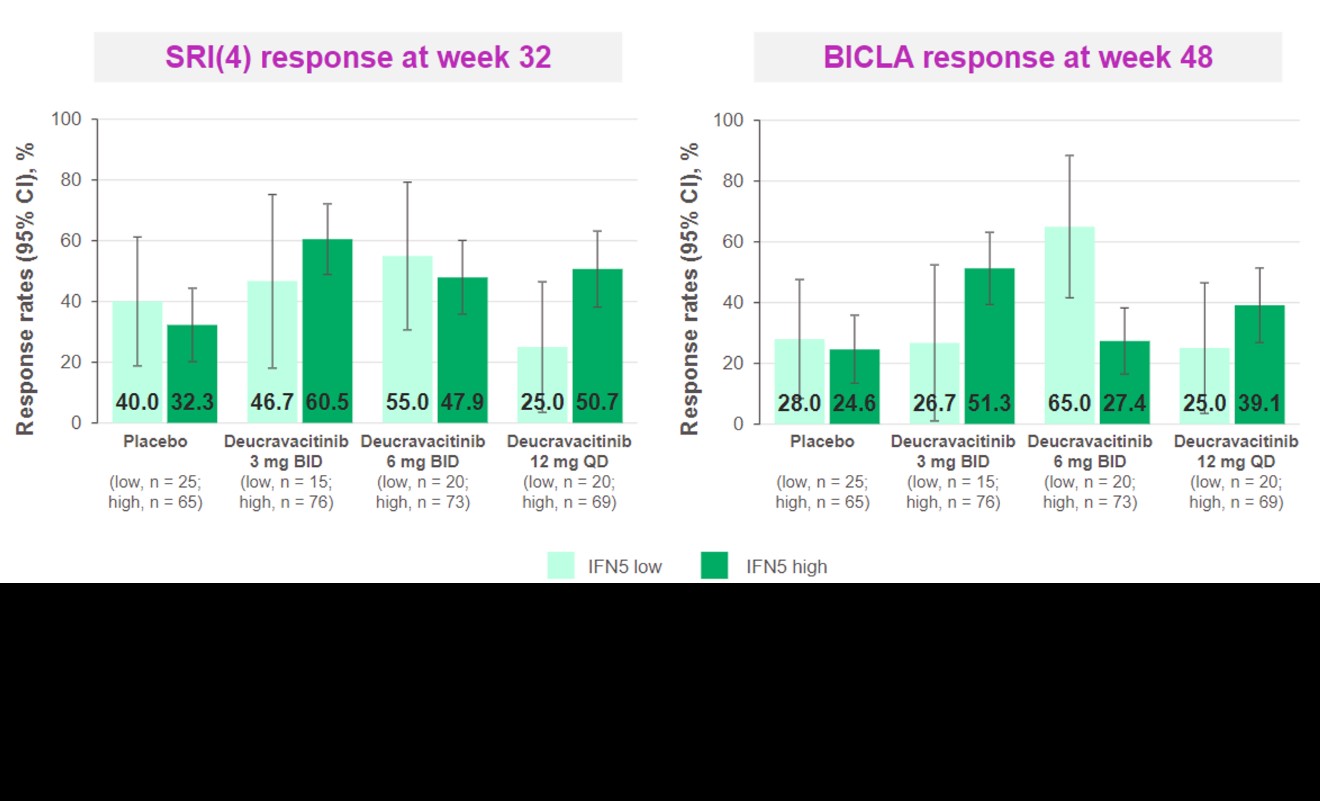
Results: An IFN 5-gene (MX1, HERC5, IFIT1, RSAD2, and EIF2AK2) signature score was identified and used to classify patients into IFN-high or IFN-low subgroups (Figure 1). Higher baseline score was associated with higher baseline SLEDAI and Cutaneous Lupus Erythematosus Disease Area and Severity Index scores, higher IFN activity biomarker (eg, IFN𝛼, IFN𝜆, B-cell activating factor, C-X-C motif chemokine ligand 10) and anti–double-stranded DNA levels, and lower complement and lymphocyte counts. Deucravacitinib reduced the IFN score from weeks 4 through 44 by > 50%. Patients with high IFN scores had numerically higher SRI(4) response rates at week 32 and BICLA response rates at week 48 in the 3-mg BID and 12-mg QD dose groups, but not in the 6-mg BID group, compared with patients with low IFN score (Figure 2).
Conclusion: These data support the IFN 5-gene signature score as a biomarker to classify patients with SLE into IFN-high or IFN-low subgroups; however, clinical response by IFN score was inconsistently improved. IFN-regulated gene expression performs well as a pharmacodynamic biomarker to confirm deucravacitinib mechanism of action and to aid in phase 3 dose selection.
Reference:
1. Morand E, et al. Arthritis Rheumatol 2023;75:242–252.
C. Wu: Bristol Myers Squibb, 3; Y. Hu: BMS, 3, 12, BMS stock holder; M. Crow: AMPEL BioSolutions, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, Eli Lilly, 2, Gilead Sciences, 5, GlaxoSmithKlein(GSK), 2; A. Saxena: AbbVie/Abbott, 1, AstraZeneca, 1, GlaxoSmithKlein(GSK), 1; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; C. Hobar: Bristol-Myers Squibb(BMS), 3; A. Coles: Bristol-Myers Squibb(BMS), 3; I. Catlett: Bristol Myers Squibb, 3, 8.
Background/Purpose: Elevated IFN activity is observed in a subset of lupus subjects suggesting that those with higher levels of IRGs may benefit from interferon targeted therapies, however, to date responses by this dichotomization have been inconsistent. Tyrosine kinase 2 (TYK2) mediates cytokine pathways (eg, type I IFN, IL-12 and IL-23) linked with SLE pathogenesis. Deucravacitinib is a first-in-class, oral, selective, allosteric TYK2 inhibitor and was found to be efficacious in a phase 2 SLE trial.1 We developed a customized IFN 5-gene signature score, assessed the pharmacodynamic effects of deucravacitinib on the IFN score, and evaluated the score's association with SLE disease activity and clinical response in the phase 2 trial (NCT03252587).
Methods: Patients were randomized equally to placebo or deucravacitinib (3 mg twice daily [BID], 6 mg BID, or 12 mg once daily [QD]). DxTerity chemical ligation-dependent probe amplification was used to measure 51 immune system–related genes from whole blood. IFN genes were selected based on distribution, correlations, hierarchical clustering, and consistency of k-means clusters. Serum proteins, blood cell subsets, and antibodies were measured by immunoassays and flow cytometry. Systemic Lupus Erythematosus Responder Index-4 (SRI[4]) and British Isles Assessment Group–based Composite Lupus Assessment (BICLA) were measured at weeks 32 and 48.
Results: An IFN 5-gene (MX1, HERC5, IFIT1, RSAD2, and EIF2AK2) signature score was identified and used to classify patients into IFN-high or IFN-low subgroups (Figure 1). Higher baseline score was associated with higher baseline SLEDAI and Cutaneous Lupus Erythematosus Disease Area and Severity Index scores, higher IFN activity biomarker (eg, IFN𝛼, IFN𝜆, B-cell activating factor, C-X-C motif chemokine ligand 10) and anti–double-stranded DNA levels, and lower complement and lymphocyte counts. Deucravacitinib reduced the IFN score from weeks 4 through 44 by > 50%. Patients with high IFN scores had numerically higher SRI(4) response rates at week 32 and BICLA response rates at week 48 in the 3-mg BID and 12-mg QD dose groups, but not in the 6-mg BID group, compared with patients with low IFN score (Figure 2).
Conclusion: These data support the IFN 5-gene signature score as a biomarker to classify patients with SLE into IFN-high or IFN-low subgroups; however, clinical response by IFN score was inconsistently improved. IFN-regulated gene expression performs well as a pharmacodynamic biomarker to confirm deucravacitinib mechanism of action and to aid in phase 3 dose selection.
Reference:
1. Morand E, et al. Arthritis Rheumatol 2023;75:242–252.

Figure 1. IFN 5-gene signature score with k-means clustering–derived cut point in the PAISLEY trial

Figure 2. Clinical response by IFN 5-gene score subgroup
C. Wu: Bristol Myers Squibb, 3; Y. Hu: BMS, 3, 12, BMS stock holder; M. Crow: AMPEL BioSolutions, 2, AstraZeneca, 2, Bristol Myers Squibb, 2, Eli Lilly, 2, Gilead Sciences, 5, GlaxoSmithKlein(GSK), 2; A. Saxena: AbbVie/Abbott, 1, AstraZeneca, 1, GlaxoSmithKlein(GSK), 1; C. Arriens: AstraZeneca, 1, 5, 6, Aurinia, 6, Bristol-Myers Squibb, 1, 5, Cabaletta, 1, GSK, 1, Kezar, 1, UCB, 1; C. Hobar: Bristol-Myers Squibb(BMS), 3; A. Coles: Bristol-Myers Squibb(BMS), 3; I. Catlett: Bristol Myers Squibb, 3, 8.




