Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Treatment I: Renal (0781–0786)
0786: A Retrospective Analysis of the Efficacy of the Euro-Lupus Nephritis Cyclophosphamide Regimen versus NIH Regimen in a South Carolina Lupus Nephritis Cohort
Sunday, November 12, 2023
3:15 PM - 3:25 PM PT
Location: Ballroom 20D
- AA
Anna Arar, MS, BS
Medical University of South Carolina
Charleston, SC, United StatesDisclosure information not submitted.
Presenting Author(s)
Anna Arar, Diane L. Kamen, Paul Nietert and Melissa Cunningham, Medical University of South Carolina, Charleston, SC
Background/Purpose: Manifestations of systemic lupus erythematous (SLE) vary in severity and presentation; lupus nephritis (LN) affects up to half of SLE patients and confers a high risk of morbidity and mortality. Since the early 1990s, the standard regimen for SLE patients with glomerulonephritis was high-dose IV cyclophosphamide (CTX) given once monthly for 6 months with 2 additional quarterly doses. This was widely accepted until the Euro-Lupus Nephritis Trial (ELNT) results were published in 2002 and demonstrated non-inferiority. It is now accepted to give a fixed dose of 500mg IV CTX every two weeks for 12 weeks (6 doses). However, the trial population in ELNT differs significantly than the population of SLE patients treated in the United States and more specifically, South Carolina, where African Americans represent 26.7% of the population. African Americans have a higher prevalence of LN and often more severe disease which may suggest the need for more aggressive therapy. Additionally, renal damage progressing to ESRD is still more common in African Americans. We hypothesized that the two regimens may not have equivalent efficacies in multiethnic patient cohorts.
Methods: We undertook a retrospective analysis to compare the Euro-Lupus Nephritis regimen to the NIH regimen in patients with lupus nephritis treated at the Medical University of South Carolina. Data was collected from patients with LN age 18-90 years old treated with CTX at MUSC from 2012 to 2022. There were no exclusion criteria. Data includes demographics, dates of visits and treatments, renal histological parameters, clinical laboratory values of lupus disease activity and renal status, medications, and dosing data.
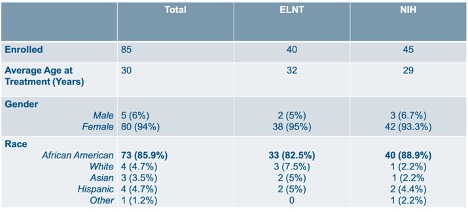
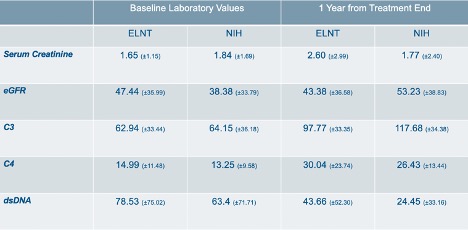
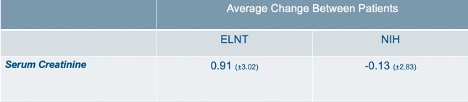
Results: Eighty-five patients fit the inclusion criteria. Of these, 40 received ELNT, and 45 received the NIH protocol. There are 80 females and 5 males. Of the 85 subjects, 73 are African American (85.9%). In the ELNT cohort, 33 are African American (82.5%). There are 40 African Americans in the NIH group (88.9%). Demographics are provided in Table 1. Baseline and one year laboratory averages with standard deviations are provided in Table 2. Table 3 shows the average change in serum Cr amongst patients in each treatment group. In the ELNT group, the average change showed an increase in creatinine by 0.91 (±3.02). The serum creatinine in the NIH cohort decreased by 0.13 (±2.83). Findings from this ongoing analysis suggest that at the one-year mark, patients in the ELNT group on average have an increase in serum creatinine, with 10 patients (25%) needing to be put on hemodialysis versus only 4 patients (8.8%) in the NIH cohort (p=0.0468).
Conclusion: No known prior studies have evaluated the efficacy of the ELNT protocol in a predominantly African American cohort. This project is currently ongoing. Preliminary results indicate that patients receiving the ELNT protocol at MUSC have increased serum creatinine at 1 year, possibly suggesting that the therapy is not aggressive enough. This is also shown in the proportion of patients requiring hemodialysis within 1 year. Additional research is needed to identify the optimal choice for cyclophosphamide regimen in different populations of patients with lupus nephritis.
A. Arar: None; D. Kamen: None; P. Nietert: None; M. Cunningham: Aurinia, 2.
Background/Purpose: Manifestations of systemic lupus erythematous (SLE) vary in severity and presentation; lupus nephritis (LN) affects up to half of SLE patients and confers a high risk of morbidity and mortality. Since the early 1990s, the standard regimen for SLE patients with glomerulonephritis was high-dose IV cyclophosphamide (CTX) given once monthly for 6 months with 2 additional quarterly doses. This was widely accepted until the Euro-Lupus Nephritis Trial (ELNT) results were published in 2002 and demonstrated non-inferiority. It is now accepted to give a fixed dose of 500mg IV CTX every two weeks for 12 weeks (6 doses). However, the trial population in ELNT differs significantly than the population of SLE patients treated in the United States and more specifically, South Carolina, where African Americans represent 26.7% of the population. African Americans have a higher prevalence of LN and often more severe disease which may suggest the need for more aggressive therapy. Additionally, renal damage progressing to ESRD is still more common in African Americans. We hypothesized that the two regimens may not have equivalent efficacies in multiethnic patient cohorts.
Methods: We undertook a retrospective analysis to compare the Euro-Lupus Nephritis regimen to the NIH regimen in patients with lupus nephritis treated at the Medical University of South Carolina. Data was collected from patients with LN age 18-90 years old treated with CTX at MUSC from 2012 to 2022. There were no exclusion criteria. Data includes demographics, dates of visits and treatments, renal histological parameters, clinical laboratory values of lupus disease activity and renal status, medications, and dosing data.
Results: Eighty-five patients fit the inclusion criteria. Of these, 40 received ELNT, and 45 received the NIH protocol. There are 80 females and 5 males. Of the 85 subjects, 73 are African American (85.9%). In the ELNT cohort, 33 are African American (82.5%). There are 40 African Americans in the NIH group (88.9%). Demographics are provided in Table 1. Baseline and one year laboratory averages with standard deviations are provided in Table 2. Table 3 shows the average change in serum Cr amongst patients in each treatment group. In the ELNT group, the average change showed an increase in creatinine by 0.91 (±3.02). The serum creatinine in the NIH cohort decreased by 0.13 (±2.83). Findings from this ongoing analysis suggest that at the one-year mark, patients in the ELNT group on average have an increase in serum creatinine, with 10 patients (25%) needing to be put on hemodialysis versus only 4 patients (8.8%) in the NIH cohort (p=0.0468).
Conclusion: No known prior studies have evaluated the efficacy of the ELNT protocol in a predominantly African American cohort. This project is currently ongoing. Preliminary results indicate that patients receiving the ELNT protocol at MUSC have increased serum creatinine at 1 year, possibly suggesting that the therapy is not aggressive enough. This is also shown in the proportion of patients requiring hemodialysis within 1 year. Additional research is needed to identify the optimal choice for cyclophosphamide regimen in different populations of patients with lupus nephritis.

Table 1: Enrollment Demographics

Table 2: Laboratory value averages with standard deviations at baseline and 1 year from last treatment.

Table 3: Average change of serum creatinine in patients between 1 year and baseline laboratory values. The increase in ELNT was 0.91 while NIH improved by an average of 0.13.
A. Arar: None; D. Kamen: None; P. Nietert: None; M. Cunningham: Aurinia, 2.



