Abstract Session
Professional development and education
Session: Abstracts: Professional Education (0763–0768)
0766: The OMERACT GCA Phantom Project: Validation of 3D Printed Ultrasound Training Models for Giant Cell Arteritis
Sunday, November 12, 2023
2:45 PM - 2:55 PM PT
Location: Room 26A-B

Valentin Sebastian Schäfer, MD
University Hospital Bonn
53127 Bonn, GermanyDisclosure information not submitted.
Presenting Author(s)
Valentin Sebastian Schäfer1, Tobias Schremmer2, Florian Recker3, Wolfgang Hartung4, Christina Duftner5, Sara Monti6, Alojzija Hocevar7, Rositsa Karalilova8, Annamaria Iagnocco9, PIERLUIGI MACCHIONI10, Giuseppe Antonio Germano11, Maria Esperanza Naredo Sanchez12, Eugenio De Miguel13, Luca Seitz14, Thomas Daikeler15, Markus Aschwanden16, Dennis Boumans17, Chetan Mukhtyar18, smith kate19, Richard Wakefield20, Andreas Diamantopoulos21, Stavros Chrysidis22, Berit Dalsgaard Nielsen23, Kresten Keller24, Uffe Dohn25, Lene Terslev26, Marcin Milchert27, Aaron Juche28, Wolfgang Schmidt29, George A. W. Bruyn30 and Christian Dejaco31, 1Clinic of Internal Medicine III, Department of Oncology, Hematology, Rheumatology and Clinical Immunology, University Hospital of Bonn, Bonn, Germany, 2Universitätsklinikum Bonn, Bonn, Germany, 3Department for Gynecology and Obstetrics, University Hospital Bonn, Bonn, Germany, 4Asklepios Clinic, Tegernheim, Germany, 5Department of Internal Medicine, Clinical Division of Internal Medicine II, Medical University Innsbruck, Innsbruck, Austria, 6Division of Rheumatology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; Department of Internal Medicine and Therapeutics, Università di Pavia, Pavia, Italy, 7Department of Rheumatology, Universitiy Medical Centre Ljubljana, Ljubljana, Slovenia, 8Clinic of Rheumatology, Medical University Plovdiv, Plovdiv, Bulgaria, 9University of Turin, Roma, Italy, 10Azienda USL -IRCCS di Reggio Emilia, Reggio Emilia, Italy, 11Rheumatology Unit- Arcispedale Santa Maria Nuova Reggio Emilia-Italy, Reggio Emilia, Italy, 12Rheumatology Department and Joint and Bone Research Unit, Hospital Universitario Fundación Jiménez Díaz, Madrid, Spain, 13Hospital Universitario La Paz, Madrid, Spain, 14Rheumatology and Immunology, Inselspital University Hospital Bern, Bern, Switzerland, 15Clinic for Rheumatology, University Hospital Basel, Basel, Switzerland, 16Department of Angiology, University Hospital Basel, Basel, Switzerland, 17Rheumatology and Clinical Immunology, Hospital Group Twente, Almelo, Netherlands, 18Vasculitis service, Rheumatology department, Norfolk and Norwich University Hospital, Norwich, United Kingdom, 19NIHR Leeds Biomedical Research Centre, Leeds, United Kingdom, 20s Leeds Insitute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 21Akershus University Hospital, Lørenskog, Norway, 22Department of Rheumatology, Southwest Jutland Hospital Esbjerg, Esbjerg, Denmark, 23Department of Rheumatology, Aarhus University Hospital, Aarhus, Denmark; Department of Medicine, The Regional Hospital in Horsens, Horsens, Denmark, 24Department of Rheumatology, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus, Denmark, 25Copenhagen University Hospital at Hvidovre, Hvidovre, Denmark, 26Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, Denmark, 27Department of Internal Medicine, Rheumatology, Diabetology, Geriatrics and Clinical Immunology, Pomeranian Medical University in Szczecin, Szczecin, Poland, 28Immanuel Krankenhaus Berlin, Medical Centre for Rheumatology Berlin-Buch, Berlin, Germany, 29Rheumatology, Immanuel Krankenhaus Berlin, Medical Centre for Rheumatology Berlin-Buch, Berlin, Germany, 30Reumakliniek Lelystad, Loenga, Netherlands, 31Azienda Sanitaria Alto Adige, Brunico, Italy
Background/Purpose: Ultrasonography has been validated as a diagnostic tool for giant cell arteritis (GCA). There is a need to develop training resources, because the glucocorticoid responsiveness of abnormalities of affected vessels and the uunpredictability and relative scarcity of presentation makes it challenging to predictably use real patients for educational purposes. To address this, we have developed cost-effective ultrasound phantoms using high-resolution 3D printing techniques [1]. The aim of this study is to validate these models within the subgroup on large vessel vasculitis of the Outcome Measures in Rheumatology (OMERACT) ultrasound working group.
Methods: Normal and pathological phantoms of the axillary (AA) and temporal artery (TA) were developed and printed using a Formlabs SLA 3D printer. The phantoms were then embedded in a special gelatine as eight sets of three (AA) or two (TA). Participants from twelve European countries were provided with eight sets of both AA and TA phantoms. The phantoms were evaluated individually in a blinded fashion using a predefined protocol and the OMERACT definitions of abnormalities in GCA [2], [3]. The artificial intima-media thickness (IMT) of each vessel phantom was measured at most prominent site in mm, preferably on the distal wall [4]. The phantoms were classified by each investigators normal (AA&TA), acute (AA&TA), chronic (AA only), or none of the above (AA&TA). Descriptive parameters and interrater-reliability were calculated. Furthermore, we conducted a one-way analysis of variance.
Results: The phantoms were correctly classified in 87% of cases resulting in a Fleiss' kappa of 0.80 (95%CI 0.78-0.82). The published cut-off values for normal and pathological IMT were met, with the exception of the parietal branch of the TA [5]. Excellent agreement among participants was demonstrated for IMT measurements, with an intraclass correlation coefficient (ICC 1.1) of 0.98 (95% CI 0.98-0.99). The results of IMT measurements are shown in Table 1. A one-way analysis of variance (ANOVA) revealed a significant difference in average IMT between pathological and healthy phantoms for both AA and TA. Detailed results of the ANOVA, divided by the different arteries, are presented in Table 2.
Conclusion: Our novel ultrasound models accurately represent the normal state and pathology of GCA adhering to the OMERACT definitions and cut-off values for IMT. There was a high agreement among investigators to correctly classify the models and good reliability with measurement of artificial IMT. This is also a first step in the evaluation of the phantom for educational purposes.
V. Schäfer: None; T. Schremmer: None; F. Recker: None; W. Hartung: None; C. Duftner: None; S. Monti: CSL Vifor, 6; A. Hocevar: None; R. Karalilova: None; A. Iagnocco: None; P. MACCHIONI: None; G. Germano: None; M. Naredo Sanchez: None; E. De Miguel: None; L. Seitz: None; T. Daikeler: None; M. Aschwanden: None; D. Boumans: None; C. Mukhtyar: None; s. kate: None; R. Wakefield: None; A. Diamantopoulos: None; S. Chrysidis: None; B. Nielsen: None; K. Keller: None; U. Dohn: None; L. Terslev: Eli Lilly, 1, Janssen, 1, 6, Novartis, 6, UCB, 1; M. Milchert: None; A. Juche: None; W. Schmidt: AbbVie/Abbott, 1, 5, 6, Amgen, 1, 6, Bristol-Myers Squibb(BMS), 6, Chugai, 6, GlaxoSmithKlein(GSK), 1, 5, 6, Janssen, 6, Medac, 6, Novartis, 1, 5, 6, Roche, 6, UCB, 6; G. Bruyn: None; C. Dejaco: AbbVie/Abbott, 2, 5, 6, Amgen, 6, Eli Lilly, 2, 6, Galapagos Pharma, 2, 6, Janssen, 1, Novartis, 2, 5, 6, Pfizer, 6, Sparrow, 2.
Background/Purpose: Ultrasonography has been validated as a diagnostic tool for giant cell arteritis (GCA). There is a need to develop training resources, because the glucocorticoid responsiveness of abnormalities of affected vessels and the uunpredictability and relative scarcity of presentation makes it challenging to predictably use real patients for educational purposes. To address this, we have developed cost-effective ultrasound phantoms using high-resolution 3D printing techniques [1]. The aim of this study is to validate these models within the subgroup on large vessel vasculitis of the Outcome Measures in Rheumatology (OMERACT) ultrasound working group.
Methods: Normal and pathological phantoms of the axillary (AA) and temporal artery (TA) were developed and printed using a Formlabs SLA 3D printer. The phantoms were then embedded in a special gelatine as eight sets of three (AA) or two (TA). Participants from twelve European countries were provided with eight sets of both AA and TA phantoms. The phantoms were evaluated individually in a blinded fashion using a predefined protocol and the OMERACT definitions of abnormalities in GCA [2], [3]. The artificial intima-media thickness (IMT) of each vessel phantom was measured at most prominent site in mm, preferably on the distal wall [4]. The phantoms were classified by each investigators normal (AA&TA), acute (AA&TA), chronic (AA only), or none of the above (AA&TA). Descriptive parameters and interrater-reliability were calculated. Furthermore, we conducted a one-way analysis of variance.
Results: The phantoms were correctly classified in 87% of cases resulting in a Fleiss' kappa of 0.80 (95%CI 0.78-0.82). The published cut-off values for normal and pathological IMT were met, with the exception of the parietal branch of the TA [5]. Excellent agreement among participants was demonstrated for IMT measurements, with an intraclass correlation coefficient (ICC 1.1) of 0.98 (95% CI 0.98-0.99). The results of IMT measurements are shown in Table 1. A one-way analysis of variance (ANOVA) revealed a significant difference in average IMT between pathological and healthy phantoms for both AA and TA. Detailed results of the ANOVA, divided by the different arteries, are presented in Table 2.
Conclusion: Our novel ultrasound models accurately represent the normal state and pathology of GCA adhering to the OMERACT definitions and cut-off values for IMT. There was a high agreement among investigators to correctly classify the models and good reliability with measurement of artificial IMT. This is also a first step in the evaluation of the phantom for educational purposes.

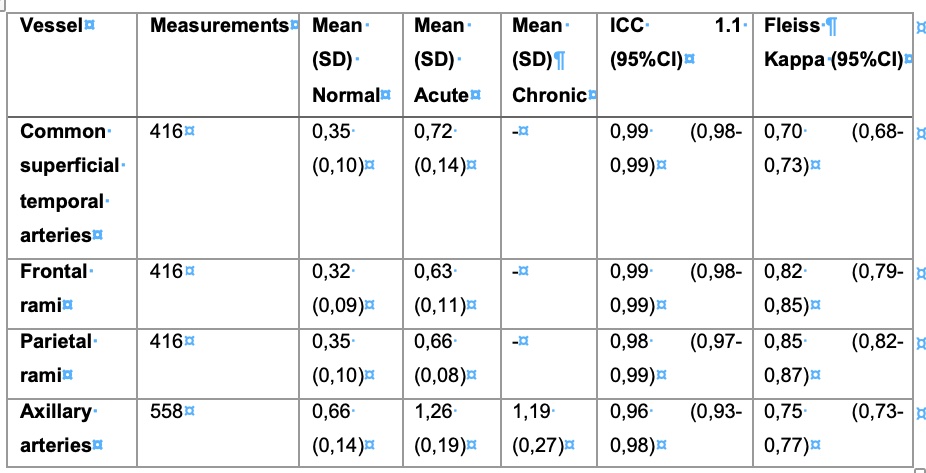
Table 1 – Results of the IMT measurements and Interrater-reliability
Notice, that there are no chronic phantoms of temporal arteries (SD: standard deviation; ICC: intra-class correlation coefficient; CI: confidence interval)
Notice, that there are no chronic phantoms of temporal arteries (SD: standard deviation; ICC: intra-class correlation coefficient; CI: confidence interval)

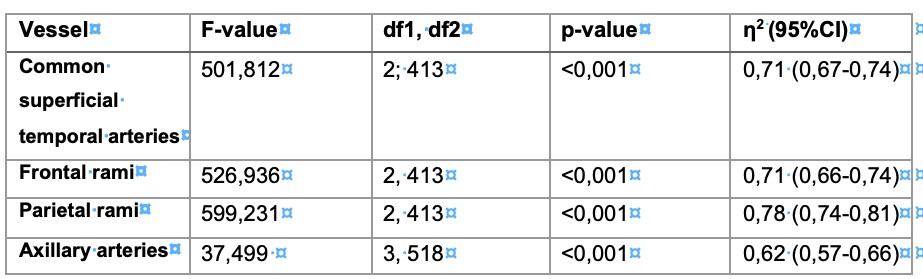
Table 2 – Results of the analysis of variance
The table shows the results of the analysis of variance along with their significance (p-value) and their effect size (η2) (p-value: probability value; η2: partial eta square; df: degrees of freedom; CI: confidence interval)
The table shows the results of the analysis of variance along with their significance (p-value) and their effect size (η2) (p-value: probability value; η2: partial eta square; df: degrees of freedom; CI: confidence interval)

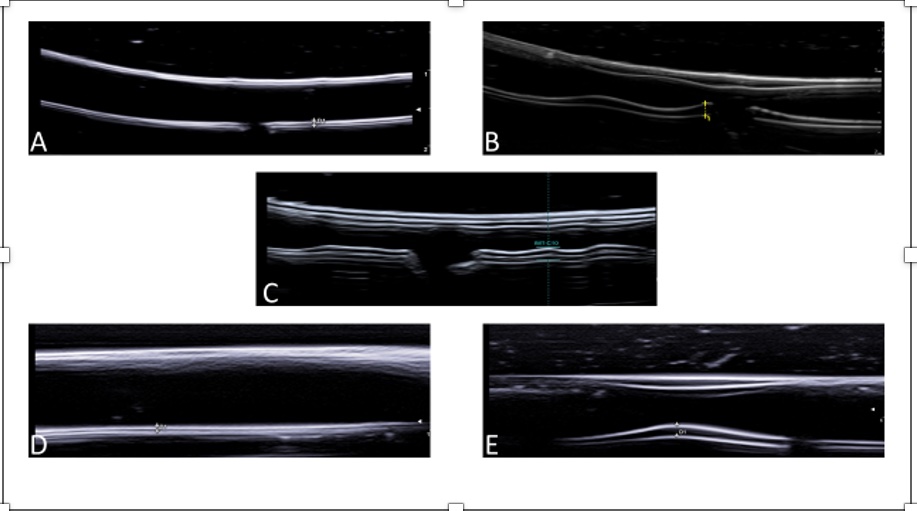
Figure 1 – Ultrasound of the phantoms
A=Normal axillary artery; B=Axillary artery with acute GCA pathology; C=Axillary artery with GCA chronic pathology; D=Normal temporal artery; E=Temporal artery with acute GCA pathology
A=Normal axillary artery; B=Axillary artery with acute GCA pathology; C=Axillary artery with GCA chronic pathology; D=Normal temporal artery; E=Temporal artery with acute GCA pathology
V. Schäfer: None; T. Schremmer: None; F. Recker: None; W. Hartung: None; C. Duftner: None; S. Monti: CSL Vifor, 6; A. Hocevar: None; R. Karalilova: None; A. Iagnocco: None; P. MACCHIONI: None; G. Germano: None; M. Naredo Sanchez: None; E. De Miguel: None; L. Seitz: None; T. Daikeler: None; M. Aschwanden: None; D. Boumans: None; C. Mukhtyar: None; s. kate: None; R. Wakefield: None; A. Diamantopoulos: None; S. Chrysidis: None; B. Nielsen: None; K. Keller: None; U. Dohn: None; L. Terslev: Eli Lilly, 1, Janssen, 1, 6, Novartis, 6, UCB, 1; M. Milchert: None; A. Juche: None; W. Schmidt: AbbVie/Abbott, 1, 5, 6, Amgen, 1, 6, Bristol-Myers Squibb(BMS), 6, Chugai, 6, GlaxoSmithKlein(GSK), 1, 5, 6, Janssen, 6, Medac, 6, Novartis, 1, 5, 6, Roche, 6, UCB, 6; G. Bruyn: None; C. Dejaco: AbbVie/Abbott, 2, 5, 6, Amgen, 6, Eli Lilly, 2, 6, Galapagos Pharma, 2, 6, Janssen, 1, Novartis, 2, 5, 6, Pfizer, 6, Sparrow, 2.



