Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Measures & Measurement of Healthcare Quality (0751–0756)
0753: Facility Variation in HLA-B*58:01 Allele Testing for Asian and Black Patients Receiving Allopurinol in the Veterans Affairs Healthcare System
Sunday, November 12, 2023
2:30 PM - 2:40 PM PT
Location: Room 25A-C
- JS
Jeremy Sullivan, MD
Arthritis and Osteoporosis Consultants of the Carolinas
Charlotte, NC, United StatesDisclosure information not submitted.
Presenting Author(s)
Jeremy Sullivan1, Anna Ware2, Gary Tarasovsky3, Cherish Wilson4, Mary Whooley3, Jasvinder Singh5, Jinoos Yazdany6 and Gabriela Schmajuk4, 1University of California San Francisco, San Francisco, CA, 2Palo Alto VA, Minneapolis, MN, 3San Francisco VA, San Francisco, CA, 4UCSF / SFVA, San Francisco, CA, 5University of Alabama at Birmingham, Birmingham, AL, 6University of California, General Department of Medicine, Division of Rheumatology, San Francisco, CA
Background/Purpose: New guidelines published in 2020 conditionally recommend HLA-B*58:01 allele testing for South Asian and Black patients receiving allopurinol to reduce the risk of severe cutaneous adverse events. However, no data exists on population-wide genetic testing rates. We investigated HLA-B*58:01 testing among allopurinol users in the Veterans Health Administration (VHA) Healthcare System.
Methods: Using data from the VHA Corporate Data Warehouse (CDW), we identified facilities with structured fields available for capturing HLA-B*58:01 test results. From these facilities, we identified all patients with a current, active prescription for allopurinol as of December 2022. We assessed the proportion of patients with a documented HLA-B*58:01 test at any time within the CDW, by race or ethnicity. We repeated this analysis among incident users of allopurinol (defined as users with a prescription in 2022 and no allopurinol use in the preceding 3 years). Finally, we examined variation in HLA-B*58:01 testing among self-identified Asian or Black patients by VHA facility, among facilities with ≥ 20 eligible patients. We contacted the top performing facility to learn about local workflows for obtaining genetic testing for allopurinol users.
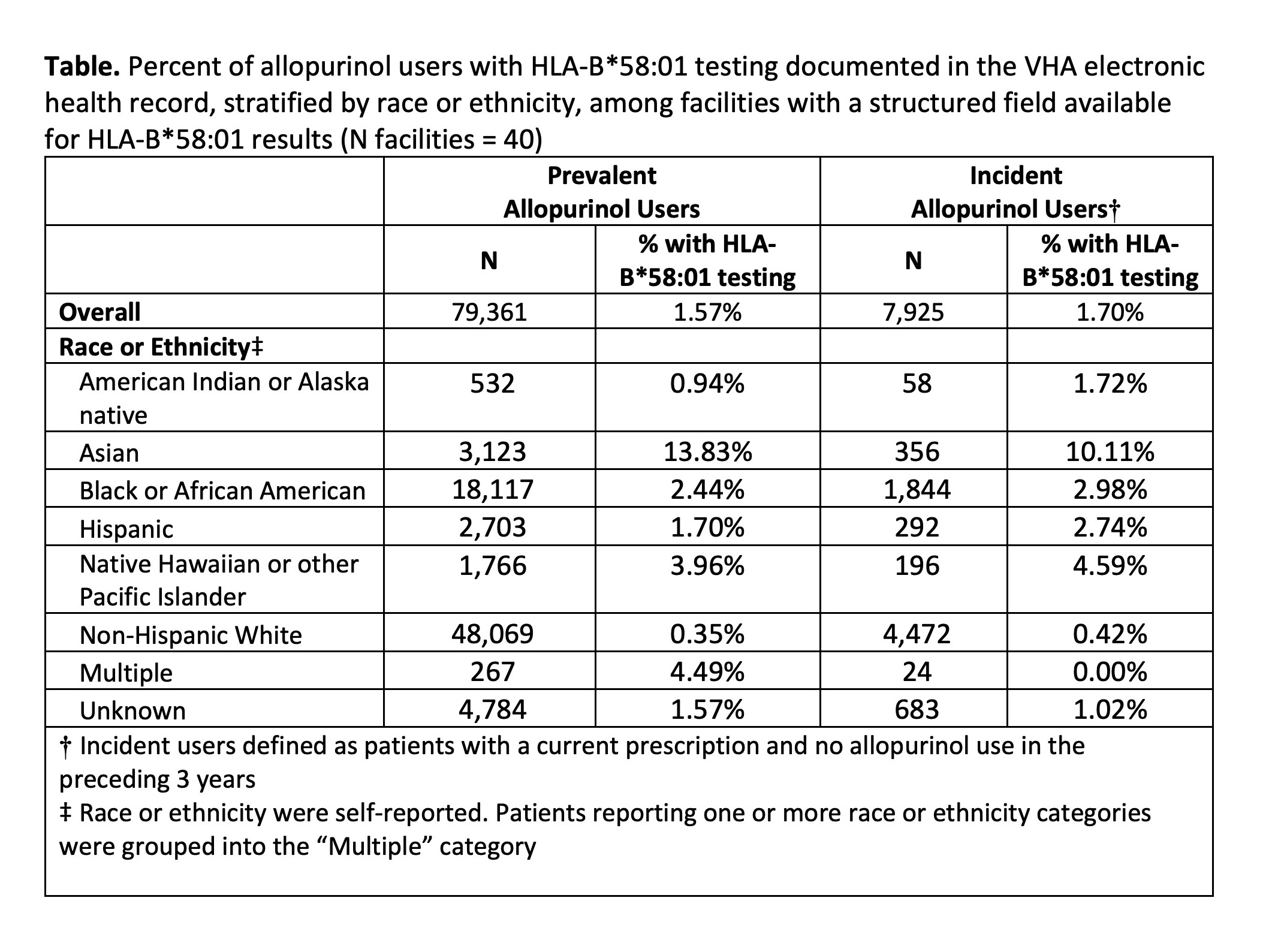
Results: Only 40 out of 130 facilities had a structured field available for capturing HLA-B*58:01 test results. We identified 79,361 users of allopurinol at these facilities; 98% of them were male, and mean age was 70 years (SD 12). Among prevalent users, 13.8% of Asian and 2.4% of Black patients were ever tested for the HLA-B*58:01 allele (Table). Among incident users, testing was documented among 10.1% of Asian and 3.0% of Black patients. We observed wide variation in HLA-B*58:01 testing across facilities (range 0% - 42.0%; Figure). The top-performing facility (proportion tested 42.0%) relayed that they had developed an allopurinol order set and pharmacy workflow that prevented allopurinol prescribing prior to completion of genetic testing.
Conclusion: Across the VHA Healthcare System, testing for HLA-B*58:01 in high-risk groups was very low, and variation across facilities was high. Most facilities lack a structured field for HLA-B*58:01 results, which hinders measurement of quality gaps. In order to improve testing rates, facilities should consider implementing order sets or pharmacy "hard stops" that require HLA-B*58:01 testing for high-risk groups.
.jpg)
J. Sullivan: None; A. Ware: None; G. Tarasovsky: None; C. Wilson: None; M. Whooley: None; J. Singh: Other, 2, 6, 11, 11, 12, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix, Charlotte’s Web, 12, Atai life sciences, kintara therapeutics, Intelligent Biosolutions, Acumen pharmaceutical, TPT Global Tech, Vaxart pharmaceuticals, Atyu biopharma, 12, speaker’s bureau of Simply Speaking, other, 12, received institutional research support from Zimmer Biomet Holdings. JAS received food and beverage payments from Intuitive Surgical Inc./Philips Elec, Other, 12, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam; J. Yazdany: AstraZeneca, 2, 5, Aurinia, 5, Gilead, 5, Pfizer, 2; G. Schmajuk: None.
Background/Purpose: New guidelines published in 2020 conditionally recommend HLA-B*58:01 allele testing for South Asian and Black patients receiving allopurinol to reduce the risk of severe cutaneous adverse events. However, no data exists on population-wide genetic testing rates. We investigated HLA-B*58:01 testing among allopurinol users in the Veterans Health Administration (VHA) Healthcare System.
Methods: Using data from the VHA Corporate Data Warehouse (CDW), we identified facilities with structured fields available for capturing HLA-B*58:01 test results. From these facilities, we identified all patients with a current, active prescription for allopurinol as of December 2022. We assessed the proportion of patients with a documented HLA-B*58:01 test at any time within the CDW, by race or ethnicity. We repeated this analysis among incident users of allopurinol (defined as users with a prescription in 2022 and no allopurinol use in the preceding 3 years). Finally, we examined variation in HLA-B*58:01 testing among self-identified Asian or Black patients by VHA facility, among facilities with ≥ 20 eligible patients. We contacted the top performing facility to learn about local workflows for obtaining genetic testing for allopurinol users.
Results: Only 40 out of 130 facilities had a structured field available for capturing HLA-B*58:01 test results. We identified 79,361 users of allopurinol at these facilities; 98% of them were male, and mean age was 70 years (SD 12). Among prevalent users, 13.8% of Asian and 2.4% of Black patients were ever tested for the HLA-B*58:01 allele (Table). Among incident users, testing was documented among 10.1% of Asian and 3.0% of Black patients. We observed wide variation in HLA-B*58:01 testing across facilities (range 0% - 42.0%; Figure). The top-performing facility (proportion tested 42.0%) relayed that they had developed an allopurinol order set and pharmacy workflow that prevented allopurinol prescribing prior to completion of genetic testing.
Conclusion: Across the VHA Healthcare System, testing for HLA-B*58:01 in high-risk groups was very low, and variation across facilities was high. Most facilities lack a structured field for HLA-B*58:01 results, which hinders measurement of quality gaps. In order to improve testing rates, facilities should consider implementing order sets or pharmacy "hard stops" that require HLA-B*58:01 testing for high-risk groups.

.jpg)
J. Sullivan: None; A. Ware: None; G. Tarasovsky: None; C. Wilson: None; M. Whooley: None; J. Singh: Other, 2, 6, 11, 11, 12, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix, Charlotte’s Web, 12, Atai life sciences, kintara therapeutics, Intelligent Biosolutions, Acumen pharmaceutical, TPT Global Tech, Vaxart pharmaceuticals, Atyu biopharma, 12, speaker’s bureau of Simply Speaking, other, 12, received institutional research support from Zimmer Biomet Holdings. JAS received food and beverage payments from Intuitive Surgical Inc./Philips Elec, Other, 12, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam; J. Yazdany: AstraZeneca, 2, 5, Aurinia, 5, Gilead, 5, Pfizer, 2; G. Schmajuk: None.



