Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Measures & Measurement of Healthcare Quality (0751–0756)
0752: Tuberculosis Screening Among New Users of a Biologic or Targeted Synthetic DMARD: Gaps in Coverage Overall and Among JAKi Initiators
Sunday, November 12, 2023
2:15 PM - 2:25 PM PT
Location: Room 25A-C
- ER
Eric Roberts, PhD, MPH
University of California, San Francisco
SF, CA, United StatesDisclosure information not submitted.
Presenting Author(s)
Eric Roberts1, Gabriela Schmajuk2 and Jinoos Yazdany1, 1University of California San Francisco, San Francisco, CA, 2UCSF / SFVA, San Francisco, CA
Background/Purpose: Screening for latent tuberculosis (TB) is recommended prior to starting biologics or targeted synthetic DMARDs (b/tsDMARDs). With a growing number of these drugs available in rheumatology, it is important to investigate whether TB screening practices have kept pace with recommendations. Using national data, we aim to describe the proportion of new users of a b/tsDMARD who were screened for TB, and evaluate potential gaps in testing by drug type, patient characteristics and practice.
Methods: Data come from RISE, an electronic health record-based registry of US rheumatology practices with linkage to Medicare Parts A, B, and D claims. Individuals in RISE with continuous Medicare enrollment in 2017 and 2018 who were new users of a qualifying b/tsDMARD in 2018 in Medicare were included. New users were defined as those with a prescription for a b/tsDMARD in 2018 but no prescription (in Medicare or RISE) in the year prior. Use of b/tsDMARDs, information on TB testing (IGRA, TST or medical exception), and patient demographics were extracted from Medicare and RISE, to gather the most comprehensive data on b/tsDMARD use and TB testing. We calculated the proportion of patients tested in either RISE or Medicare claims in the year prior to the new b/tsDMARD, consistent with the Quality Payment Program measure. In a sensitivity analysis we permitted TB screening in the 3 years before up to 30 days after the patient's start date. We computed screening rates by b/tsDMARD type. Using logistic regression we tested for differences in screening by age, sex, race-ethnicity, insurance and socioeconomic status (using the area deprivation index). We calculated the proportion of patients screened among practices with at least 20 new users.
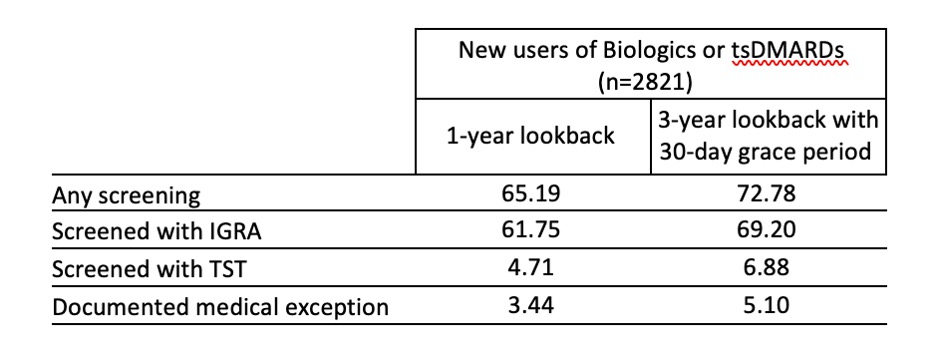
Results: Among 2821 new b/tsDMARD users, mean age was 73±8.6 years, 72% female, 73% non-Hispanic White, 6.8% Black, 4.7% Hispanic and 1.2% Asian. In the year prior to drug starts, 65.2% of patients had any TB screening documented, including 61.8% with an IGRA, 4.7% with a TST, and 3.4% with a documented medical exception. In our sensitivity analysis, 77.9% had any TB screening (Table 1). Rates of screening within 1 year by drug type were greater or equal to the overall screening rate for most drugs with the exception of JAKi (53.5%) and IL17i (10.4%) (Figure 1). In a fully adjusted logistic regression, patients in the highest quartile of SES had significantly lower odds (OR=0.64; 95%CI 0.48-0.87) of being screened within 1 year compared to the lowest quartile. 50 practices had at least 20 patients; screening rates ranged from 20-96.3% of patients (Figure 2).
Conclusion: Using a comprehensive data source that includes both registry data and Medicare claims, we found that just over 1 in 3 new users of a b/tsDMARD did not receive TB screening within the recommended time window. When the screening window was expanded, performance only slightly improved with approximately 1 in 4 new users not receiving screening. Although lower TB screening rates for IL-17i may reflect greater uncertainty about TB risk with this drug mechanism, emerging evidence suggests that TB risk for JAKi is significant. Filling these gaps in care are important areas for quality improvement.
.jpg)
.jpg)
E. Roberts: None; G. Schmajuk: None; J. Yazdany: Astra Zeneca, 2, 5, Aurinia, 5, Gilead, 5, Pfizer, 2.
Background/Purpose: Screening for latent tuberculosis (TB) is recommended prior to starting biologics or targeted synthetic DMARDs (b/tsDMARDs). With a growing number of these drugs available in rheumatology, it is important to investigate whether TB screening practices have kept pace with recommendations. Using national data, we aim to describe the proportion of new users of a b/tsDMARD who were screened for TB, and evaluate potential gaps in testing by drug type, patient characteristics and practice.
Methods: Data come from RISE, an electronic health record-based registry of US rheumatology practices with linkage to Medicare Parts A, B, and D claims. Individuals in RISE with continuous Medicare enrollment in 2017 and 2018 who were new users of a qualifying b/tsDMARD in 2018 in Medicare were included. New users were defined as those with a prescription for a b/tsDMARD in 2018 but no prescription (in Medicare or RISE) in the year prior. Use of b/tsDMARDs, information on TB testing (IGRA, TST or medical exception), and patient demographics were extracted from Medicare and RISE, to gather the most comprehensive data on b/tsDMARD use and TB testing. We calculated the proportion of patients tested in either RISE or Medicare claims in the year prior to the new b/tsDMARD, consistent with the Quality Payment Program measure. In a sensitivity analysis we permitted TB screening in the 3 years before up to 30 days after the patient's start date. We computed screening rates by b/tsDMARD type. Using logistic regression we tested for differences in screening by age, sex, race-ethnicity, insurance and socioeconomic status (using the area deprivation index). We calculated the proportion of patients screened among practices with at least 20 new users.
Results: Among 2821 new b/tsDMARD users, mean age was 73±8.6 years, 72% female, 73% non-Hispanic White, 6.8% Black, 4.7% Hispanic and 1.2% Asian. In the year prior to drug starts, 65.2% of patients had any TB screening documented, including 61.8% with an IGRA, 4.7% with a TST, and 3.4% with a documented medical exception. In our sensitivity analysis, 77.9% had any TB screening (Table 1). Rates of screening within 1 year by drug type were greater or equal to the overall screening rate for most drugs with the exception of JAKi (53.5%) and IL17i (10.4%) (Figure 1). In a fully adjusted logistic regression, patients in the highest quartile of SES had significantly lower odds (OR=0.64; 95%CI 0.48-0.87) of being screened within 1 year compared to the lowest quartile. 50 practices had at least 20 patients; screening rates ranged from 20-96.3% of patients (Figure 2).
Conclusion: Using a comprehensive data source that includes both registry data and Medicare claims, we found that just over 1 in 3 new users of a b/tsDMARD did not receive TB screening within the recommended time window. When the screening window was expanded, performance only slightly improved with approximately 1 in 4 new users not receiving screening. Although lower TB screening rates for IL-17i may reflect greater uncertainty about TB risk with this drug mechanism, emerging evidence suggests that TB risk for JAKi is significant. Filling these gaps in care are important areas for quality improvement.

Table 1. Percent of new b/tsDMARD users screened for TB within 1 year and 3 years with a 30 day grace period overall and by TB screening method.
.jpg)
Figure 1. Proportion of new b/tsDMARD users screened for TB by DMARD type.
.jpg)
Figure 2. Proportion of new b/tsDMARD users screened for TB by practice among practices with at least 20 patients.
E. Roberts: None; G. Schmajuk: None; J. Yazdany: Astra Zeneca, 2, 5, Aurinia, 5, Gilead, 5, Pfizer, 2.



