Abstract Session
Imaging
Session: Abstracts: Imaging of Rheumatic Diseases (0745–0750)
0746: The Association Between Sonographic Imaging Phenotype and Response to Treatment in Patients with Psoriatic Arthritis
Sunday, November 12, 2023
2:15 PM - 2:25 PM PT
Location: Room 24A-C
- JG
Jessica Gutierrez Manjarrez, MD
Women’s College Research Institute / Department of Medicine, University of Toronto
Toronto, ON, CanadaDisclosure information not submitted.
Presenting Author(s)
Jessica Gutierrez Manjarrez1, Sydney Thib2, Richard J. Cook3 and Lihi Eder4, 1Women’s College Research Institute / Department of Medicine, University of Toronto, Toronto, ON, Canada, 2Women’s College Research Institute, Toronto, ON, Canada, 3Department of Statistics and Actuarial Science, University of Waterloo, Waterloo, ON, Canada, 4Women’s College Research Institute, Division of Rheumatology, University of Toronto, Toronto, ON, Canada
Background/Purpose: Ultrasound (US) may improve characterization of psoriatic arthritis (PsA) phenotypes and help predict disease outcomes. We aimed to assess whether US phenotypes are associated with clinical features and treatment outcomes in patients with active PsA.
Methods: We conducted a prospective cohort study of patients with active PsA prior to initiation of systemic therapy. Disease activity in various PsA domains was clinically assessed.
Baseline US assessment of inflammatory and structural lesions was conducted for the following features: synovitis, peritenonitis, tenosynovitis, new bone formation (NBF), bone erosion, and enthesitis for inflammatory (inflm) and structural (str) lesions. The following treatment outcomes were analyzed: drug persistence, Disease Activity in PsA (DAPSA) change, and DAPSA low disease activity (LDA) at 3-6 months.
The correlation between various baseline clinical and US features was assessed (r >0.3 was considered moderate correlation). The association between baseline US features and treatment outcomes were assessed using Cox proportional hazards models (for drug persistence), linear models via GEE (for DAPSA change) and logistic GEE models (for DAPSA-LDA). Models were adjusted for type of drug (targeted (t) vs. conventional DMARDs) and prior exposure to tDMARDs.
Results: A total of 135 treatment periods (107 patients) with PsA (49% females) were analyzed. The mean age and disease duration were 47.7 (SD=13.7) and 4.7 (SD=6.8) years, respectively.
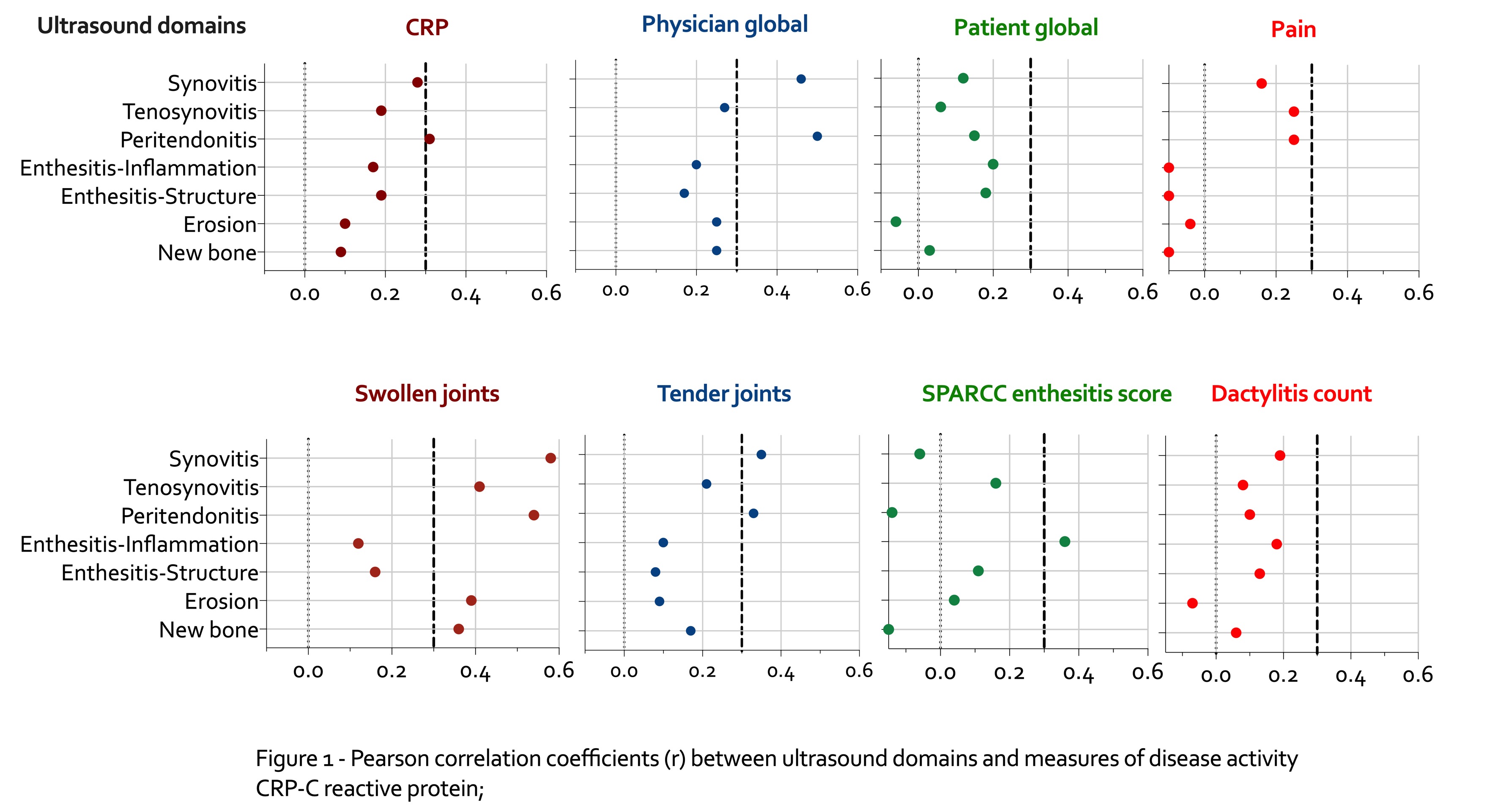
Highest correlations were found for US synovitis and peritonitis with physician global assessment (PhyGA) and swollen joints (Figure 1). PhyGA correlation with other US features was low. Synovitis and peritenonitis US scores correlated moderately with CRP and tender joints. Tenosynovitis and enthesitis-inflm showed moderate correlation with swollen joints and enthesitis score, respectively.
Drug persistence was analyzed in 105 treatment periods (40% drug discontinuation). The only US feature associated with drug discontinuation was US erosion score (adjusted Hazards Ratio 1.28, 95% confidence interval 1.03, 1.61).
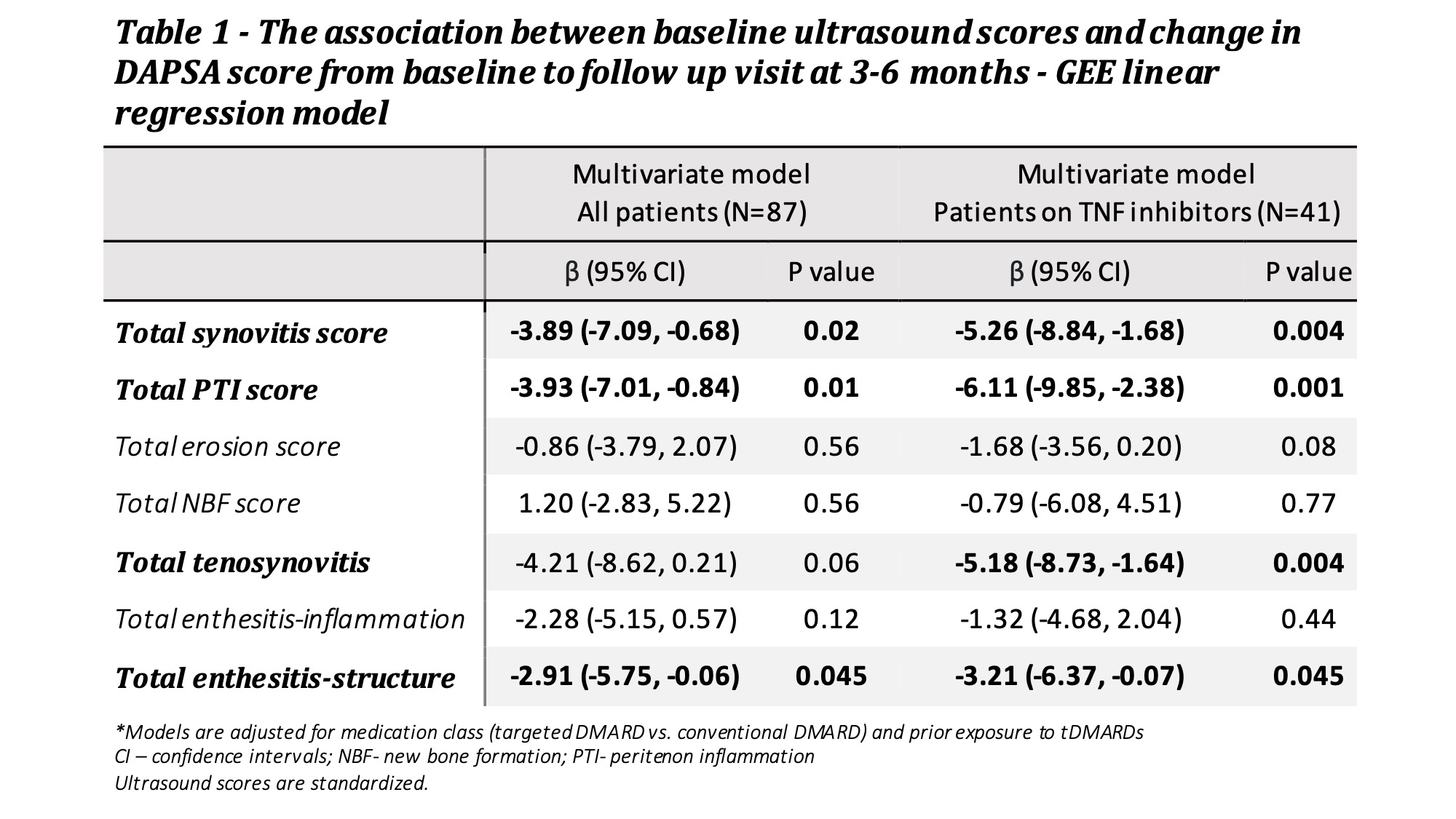
87 treatment periods were analyzed for DAPSA response at 3-6 months (54% achieved DAPSA-LDA). No association was found between any US feature and DAPSA-LDA. Greater reduction in DAPSA scores was associated with higher baseline synovitis, peritenonitis and enthesitis-str scores (Table 1). Restriction of the analysis to users of TNFi (N=41) resulted in numerically higher effect sizes for several US features.
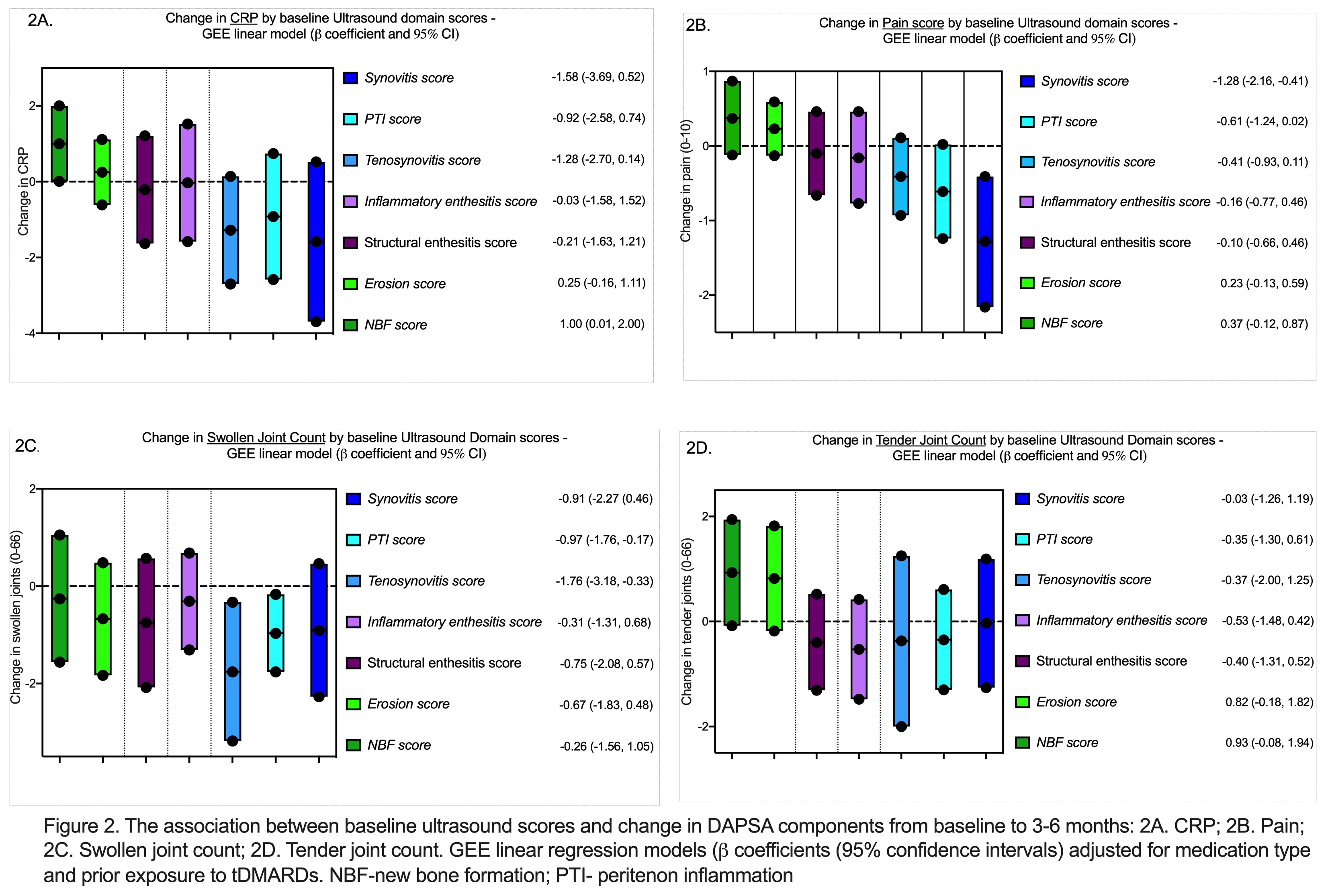
A multivariable analysis was performed to assess which components of DAPSA are influenced by US features (Figure 2). We found significant associations between US synovitis and reduction in pain (b= -1.28), US NBF and increase in CRP (b= 1.00), US tenosynovitis (b= -1.76) and peritenonitis (b= -0.97) and reduction in swollen joint count. None of the US features were associated with changes in tender joint count.
Conclusion: Discordance between imaging and clinical features of PsA exists. Sonographic synovitis, peritenonitis and tenosynovitis correlate more strongly with clinical features of disease activity and response outcomes.
J. Gutierrez Manjarrez: None; S. Thib: None; R. Cook: None; L. Eder: AbbVie, 2, 5, Eli Lilly, 2, 5, Fresenius Kabi, 5, Janssen, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, Sandoz, 5, UCB, 2, 5.
Background/Purpose: Ultrasound (US) may improve characterization of psoriatic arthritis (PsA) phenotypes and help predict disease outcomes. We aimed to assess whether US phenotypes are associated with clinical features and treatment outcomes in patients with active PsA.
Methods: We conducted a prospective cohort study of patients with active PsA prior to initiation of systemic therapy. Disease activity in various PsA domains was clinically assessed.
Baseline US assessment of inflammatory and structural lesions was conducted for the following features: synovitis, peritenonitis, tenosynovitis, new bone formation (NBF), bone erosion, and enthesitis for inflammatory (inflm) and structural (str) lesions. The following treatment outcomes were analyzed: drug persistence, Disease Activity in PsA (DAPSA) change, and DAPSA low disease activity (LDA) at 3-6 months.
The correlation between various baseline clinical and US features was assessed (r >0.3 was considered moderate correlation). The association between baseline US features and treatment outcomes were assessed using Cox proportional hazards models (for drug persistence), linear models via GEE (for DAPSA change) and logistic GEE models (for DAPSA-LDA). Models were adjusted for type of drug (targeted (t) vs. conventional DMARDs) and prior exposure to tDMARDs.
Results: A total of 135 treatment periods (107 patients) with PsA (49% females) were analyzed. The mean age and disease duration were 47.7 (SD=13.7) and 4.7 (SD=6.8) years, respectively.
Highest correlations were found for US synovitis and peritonitis with physician global assessment (PhyGA) and swollen joints (Figure 1). PhyGA correlation with other US features was low. Synovitis and peritenonitis US scores correlated moderately with CRP and tender joints. Tenosynovitis and enthesitis-inflm showed moderate correlation with swollen joints and enthesitis score, respectively.
Drug persistence was analyzed in 105 treatment periods (40% drug discontinuation). The only US feature associated with drug discontinuation was US erosion score (adjusted Hazards Ratio 1.28, 95% confidence interval 1.03, 1.61).
87 treatment periods were analyzed for DAPSA response at 3-6 months (54% achieved DAPSA-LDA). No association was found between any US feature and DAPSA-LDA. Greater reduction in DAPSA scores was associated with higher baseline synovitis, peritenonitis and enthesitis-str scores (Table 1). Restriction of the analysis to users of TNFi (N=41) resulted in numerically higher effect sizes for several US features.
A multivariable analysis was performed to assess which components of DAPSA are influenced by US features (Figure 2). We found significant associations between US synovitis and reduction in pain (b= -1.28), US NBF and increase in CRP (b= 1.00), US tenosynovitis (b= -1.76) and peritenonitis (b= -0.97) and reduction in swollen joint count. None of the US features were associated with changes in tender joint count.
Conclusion: Discordance between imaging and clinical features of PsA exists. Sonographic synovitis, peritenonitis and tenosynovitis correlate more strongly with clinical features of disease activity and response outcomes.

Figure 1 - Pearson correlation coefficients (r) between ultrasound domains and measures of disease activity

Table 1 - The association between baseline ultrasound scores and change in DAPSA score from baseline to follow up visit at 3-6 months - GEE linear regression model

Figure 2 - The association between baseline ultrasound scores and change in DAPSA components from baseline to 3-6 months: 2A. CRP; 2B. Pain; 2C. Swollen joint count; 2D. Tender joint count. GEE linear models adjusted for medication type and exposure to tDMARDs
J. Gutierrez Manjarrez: None; S. Thib: None; R. Cook: None; L. Eder: AbbVie, 2, 5, Eli Lilly, 2, 5, Fresenius Kabi, 5, Janssen, 2, 5, Novartis, 2, 5, Pfizer Inc, 2, 5, Sandoz, 5, UCB, 2, 5.



