Late-Breaking Abstract Session
Crystal arthropathies
Session: Late-Breaking Abstracts (L15–L20)
L15: AR882, an Efficacious and Selective URAT1 Inhibitor for Patients with Chronic Gouty Arthritis and Subcutaneous Tophi: Results from a Global, Prospective, Proof-of-Concept Trial Using Dual Energy Computed Tomography
Wednesday, November 15, 2023
7:30 AM - 7:45 AM PT
Location: Room 6A-B
- RK
Robert T. Keenan, MD, MPH, MBA
Arthrosi Therapeutics
Chapel Hill, NC, United StatesDisclosure information not submitted.
Presenting Author(s)
Robert Keenan1, James Cheng-Chung WEI2, Sarah Morris3, Pamella Mundell3, Wen Wei3, Ke Shi3, Zancong Shen3, Vijay Hingorani4, Shunqi Yan3, Bahram Kiani5 and Litain Yeh3, 1Arthrosi Therapeutics, Inc., Chapel Hill, NC, 2Chung Shan Medical University, Taichung, Taiwan (Republic of China), 3Arthrosi Therapeutics, Inc., San Diego, CA, 4Vanguard Healthsciences, Inc., San Diego, CA, 5Wake Forest University School of Medicine, Winston-Salem, NC
Background/Purpose: AR882 is a novel and selective URAT1 inhibitor currently in clinical stage development for the treatment of gout and tophaceous gout and has demonstrated robust and sustained serum urate (sUA) lowering in a 3-month Phase 2b trial. This proof-of-concept trial evaluates the effect of AR882 versus allopurinol on the reduction of clinically visible tophi in patients with gout using caliper measurements and Dual Energy Computer Tomography (DECT).
Methods: The phase 2 global trial recruited 42 gout patients with subcutaneous tophi. The patients were randomized into three treatment groups to receive once-daily AR882 75 mg, once-daily AR882 50 mg + allopurinol, or once-daily allopurinol up to 300 mg at a 1:1:1 ratio. Tophi measurements with calipers were completed every 4 weeks for 6 months; patients were imaged with DECT at baseline and 6 months. The primary efficacy endpoint was sUA change at month 3. Secondary endpoints included resolution of target tophus area and change from baseline in target tophus crystal volume at month 6. Safety assessments, including vital signs and electrocardiograms, were collected throughout the study.
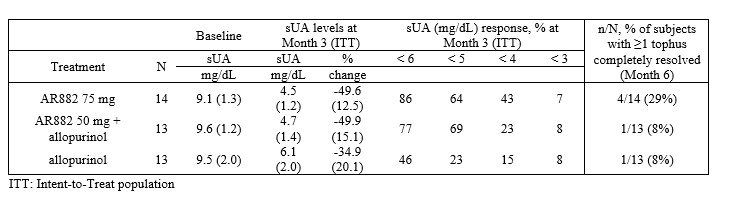
Results: In the Intent-to-Treat population, the mean baseline sUA level ranged between 9.1-9.6 mg/dL across treatment groups. At month 3, the mean sUA levels were reduced to 4.5 (±1.2), 4.7 (±1.4), and 6.1 (±2.0) mg/dL, respectively for 75 mg, 50 mg + allopurinol and allopurinol. In the 75 mg AR882 group, 86% and 64% of patients achieved sUA < 6 and < 5 mg/dL, respectively; in the 50 mg AR882 + allopurinol group, 77% and 69% of patients achieved sUA levels < 6 and < 5 mg/dL, respectively. This was compared to patients in the allopurinol group, where 46% and 23% of patients achieved sUA levels < 6 and < 5 mg/dL, respectively (Table 1).
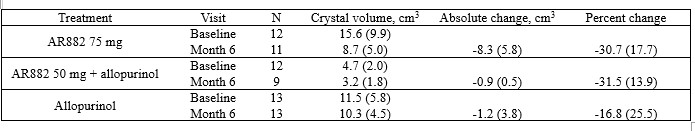
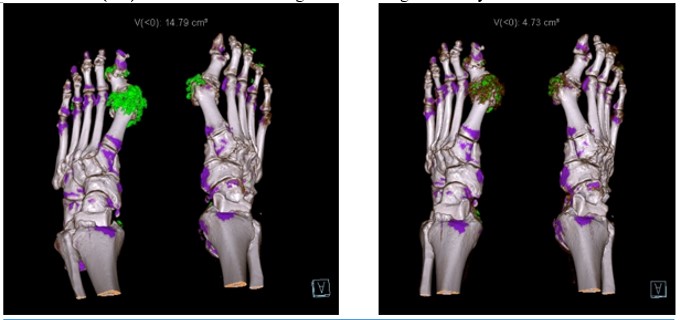
At Month 6, 4 patients (29%) in the AR882 75 mg group showed complete resolution of at least one tophus, compared to 1 patient (8%) in AR882 50 mg + allopurinol and 1 patient (8%) in allopurinol groups. The AR882 75 mg group showed a greater reduction of total urate crystal volume (-30.7%, -8.3 cm3) vs. combination (-31.5%, -0.9 cm3) or allopurinol (-16.8%, -1.2 cm3) from baseline to month 6 on DECT (Table 2). Figure 1 shows a 68% decrease in total urate crystal volume of the feet and ankles after AR882 75 mg once daily treatment for 6 months.
There were no serious adverse events related to AR882 treatment. No kidney or liver abnormalities were observed. The most frequently reported adverse event was gout flare, mild or moderate adverse events including diarrhea, headache, and upper respiratory infection. Gout flare rate appeared less in the AR882 treatment groups than in the allopurinol group.
Conclusion: In this global study, six-month treatment of AR882 demonstrated safe and efficacious sUA lowering, tophus resolution and total crystal volume dissolution in gout patients with various demographics and baseline characteristics. AR882 may offer improved efficacy and better safety compared to existing therapies in the treatment of patients with gout, including those with both clinically visible and subclinical crystal deposition.
AR882, an Efficacious and Selective URAT1 Inhibitor for Patients with Chronic Gouty Arthritis and Subcutaneous Tophi: Results from a Global, Prospective, Proof-of-Concept Trial Using Dual Energy Computed Tomography
R. Keenan: Arthrosi Therapeutics, 3; J. WEI: Arthrosi Therapeutics, 5; S. Morris: Arthrosi Therapeutics, 3; P. Mundell: Arthrosi Therapeutics, 3; W. Wei: Arthrosi Therapeutics, 3; K. Shi: Arthrosi Therapeutics, 3; Z. Shen: Arthrosi Therapeutics, 3, 3; V. Hingorani: Arthrosi Therapeutics, 2; S. Yan: Arthrosi Therapeutics, 3, 8; B. Kiani: None; L. Yeh: Arthrosi Therapeutics, 3, 8.
Background/Purpose: AR882 is a novel and selective URAT1 inhibitor currently in clinical stage development for the treatment of gout and tophaceous gout and has demonstrated robust and sustained serum urate (sUA) lowering in a 3-month Phase 2b trial. This proof-of-concept trial evaluates the effect of AR882 versus allopurinol on the reduction of clinically visible tophi in patients with gout using caliper measurements and Dual Energy Computer Tomography (DECT).
Methods: The phase 2 global trial recruited 42 gout patients with subcutaneous tophi. The patients were randomized into three treatment groups to receive once-daily AR882 75 mg, once-daily AR882 50 mg + allopurinol, or once-daily allopurinol up to 300 mg at a 1:1:1 ratio. Tophi measurements with calipers were completed every 4 weeks for 6 months; patients were imaged with DECT at baseline and 6 months. The primary efficacy endpoint was sUA change at month 3. Secondary endpoints included resolution of target tophus area and change from baseline in target tophus crystal volume at month 6. Safety assessments, including vital signs and electrocardiograms, were collected throughout the study.
Results: In the Intent-to-Treat population, the mean baseline sUA level ranged between 9.1-9.6 mg/dL across treatment groups. At month 3, the mean sUA levels were reduced to 4.5 (±1.2), 4.7 (±1.4), and 6.1 (±2.0) mg/dL, respectively for 75 mg, 50 mg + allopurinol and allopurinol. In the 75 mg AR882 group, 86% and 64% of patients achieved sUA < 6 and < 5 mg/dL, respectively; in the 50 mg AR882 + allopurinol group, 77% and 69% of patients achieved sUA levels < 6 and < 5 mg/dL, respectively. This was compared to patients in the allopurinol group, where 46% and 23% of patients achieved sUA levels < 6 and < 5 mg/dL, respectively (Table 1).
At Month 6, 4 patients (29%) in the AR882 75 mg group showed complete resolution of at least one tophus, compared to 1 patient (8%) in AR882 50 mg + allopurinol and 1 patient (8%) in allopurinol groups. The AR882 75 mg group showed a greater reduction of total urate crystal volume (-30.7%, -8.3 cm3) vs. combination (-31.5%, -0.9 cm3) or allopurinol (-16.8%, -1.2 cm3) from baseline to month 6 on DECT (Table 2). Figure 1 shows a 68% decrease in total urate crystal volume of the feet and ankles after AR882 75 mg once daily treatment for 6 months.
There were no serious adverse events related to AR882 treatment. No kidney or liver abnormalities were observed. The most frequently reported adverse event was gout flare, mild or moderate adverse events including diarrhea, headache, and upper respiratory infection. Gout flare rate appeared less in the AR882 treatment groups than in the allopurinol group.
Conclusion: In this global study, six-month treatment of AR882 demonstrated safe and efficacious sUA lowering, tophus resolution and total crystal volume dissolution in gout patients with various demographics and baseline characteristics. AR882 may offer improved efficacy and better safety compared to existing therapies in the treatment of patients with gout, including those with both clinically visible and subclinical crystal deposition.

Table 1. Mean (SD) sUA reduction, response rates and complete resolution of at least one target tophus.

Table 2. Mean (SE) change in DECT crystal volumes at Month 6.

Figure 1. DECT image of gouty arthritis showing 68% reduction in total urate crystal volume from baseline (left) to Month 6 after taking AR882 75 mg once daily.
AR882, an Efficacious and Selective URAT1 Inhibitor for Patients with Chronic Gouty Arthritis and Subcutaneous Tophi: Results from a Global, Prospective, Proof-of-Concept Trial Using Dual Energy Computed Tomography
R. Keenan: Arthrosi Therapeutics, 3; J. WEI: Arthrosi Therapeutics, 5; S. Morris: Arthrosi Therapeutics, 3; P. Mundell: Arthrosi Therapeutics, 3; W. Wei: Arthrosi Therapeutics, 3; K. Shi: Arthrosi Therapeutics, 3; Z. Shen: Arthrosi Therapeutics, 3, 3; V. Hingorani: Arthrosi Therapeutics, 2; S. Yan: Arthrosi Therapeutics, 3, 8; B. Kiani: None; L. Yeh: Arthrosi Therapeutics, 3, 8.




