Poster Session B
Rheumatoid arthritis (RA)
Session: (1308–1344) RA – Treatment Poster II
1319: Real World Data on Antifibrotics in Rheumatoid Arthritis-Interstitial Lung Disease. National Multicenter Study of 73 Patients
Monday, November 13, 2023
9:00 AM - 11:00 AM PT
Location: Poster Hall
- BA
Belén Atienza-Mateo, MD, PhD
Division of Rheumatology, Hospital Universitario Marqués de Valdecilla. IDIVAL, Immunopathology group
Santander, Cantabria, SpainDisclosure information not submitted.
Abstract Poster Presenter(s)
Belén Atienza-Mateo1, Ana Serrano-Combarro2, Maria Martin-Lopez3, Santos Castañeda4, Rafael Benito Melero-Gonzalez5, Jesus Loarce-Martos6, Natalia Mena Vazquez7, Carmen carrasco-Cubero8, Carolina Diez-Morrondo9, David Castro-Corredor10, Tomás Vazquez-Rodriguez11, andrea Garcia-Valle12, Gema Bonilla13, Marina Rodriguez-lopez14, Ignacio Brana Abascal15, SARA MARIA ROJAS HERRERA16, Juan Camilo Sarmiento-Monroy17, Pablo Andujar-Brazal18, Juan Ramon De Dios19, Carmen Gonzalez-Montagut Gomez20, Sergio Ordonez-Palau21, Anahy Maria Brandy-Garcia22, Fernando Lozano23, Maria Lopez-Lasanta24, Cristina Campos Fernández25, Marta Garijo Bufort26, Ivette Casafont-Sole27, Calderon Goercke28, Carlota Laura Iñiguez29, Francisco Ortiz-Sanjuán30, Emilio Giner-Serret31, Bryan Josue Flores Robles32, Mireia Moreno33, Evelin Cecilia Cervantes-Perez34, Diego Ferrer35, Ricardo Blanco36 and On behalf of the Collaborative Group Members37, 1Rheumatology, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 2Division of Rheumatology, Hospital Universitario Marqués de Valdecilla. IDIVAL, Immunopathology group, Santander, Spain, 3Hospital 12 de Octubre, Madrid, Spain, 4Hospital Universitario de la Princesa, Madrid, Spain, 5CHU Vigo, O Carballino, Spain, 6Ramón y Cajal University Hospital, Madrid, Spain, 7IBIMA, Málaga, Spain, 8Department of Rheumatology, Hospital Universitario de Badajoz, Badajoz, Spain, 9Division of Rheumatology, Hospital de León, León, Spain, 10General University Hospital of Ciudad Real, Santa Cruz de Mudela (Ciudad Real), Spain, 11Rheumatology, Complejo Hospitalario Universitario de Ferrol, A Coruña, Spain, 12Division of Rheumatology, Complejo Asistencial Universitario de Palencia, Palencia, Spain, 13Department of Rheumatology, Hospital Clínico Universitario La Paz, Madrid, Spain, 14Division of Rheumatology, Hospital Clínico Universitario de Santiago, La Coruna, Spain, 15Division of Rheumatology, Hospital Universitario Central de Asturias, Oviedo, Spain, 16Division of Rheumatology, Hospital de Mérida, Mérida, Spain, 17Hospital Clínic, Barcelona, Spain, 18Division of Rheumatology, Hospital Universitario Doctor Peset, Valencia, Spain, 19Hospital Universitario Araba, Vitoria, Spain, 20H. Clínico Universitario de Valladolid, Valladolid, Spain, 21H. Arnau de Vilanova, Lleida, Spain, 22Hospital Germans Trias i Pujol, Badalona, Spain, 23Hospital Universitario 12 de Octubre, Madrid, Spain, 24Hospital Universitari Vall d'Hebron, Rheumatology, Barcelona, Spain, 25Hospital General Universitario Valencia, Valencia, Spain, 26H. de Sagunto, Valencia, Italy, 27Hospital Universitari Germans Trias i Pujol, Badalona, Spain, 28Hospital Universitario Marqués de Valdecilla, Santander, Spain, 29Hospital Universitario Lucus Augusti, Lugo, Spain, 30Hospital Universitario y Politécnico La Fe, Valencia, Spain, 31Hospital Royo Villanova, Teruel, Spain, 32Hospital Universitario San Pedro, Logroño, Spain, 33Parc Tauli Hospital Universitari, I3PT(UAB), Barcelona, Spain, 34Complexo Hospitalario Universitario de Pontevedra, Pontevedra, Spain, 35Division of Pneumology, Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 36Hospital Universitario Marqués de Valdecilla, IDIVAL, Santander, Spain, 37Spanish Collaborative Group of Interstitial Lung Disease Associated with Rheumatoid Arthritis, Spain, Spain
Background/Purpose: Interstitial lung disease (ILD) is a critical complication of rheumatoid arthritis (RA). Abatacept and rituximab are the preferred disease-modifying antirheumatic drugs (DMARDs) for RA-ILD. However, progression of ILD despite its use is not uncommon. A subgroup analysis of the INBUILD trial has shown a slower decline in forced vital capacity (FVC) in patients with progressive fibrosing autoimmune disease-related ILD with nintedanib (NINTE) [Arthritis Rheumatol 2022;74(6):1039-47]. However, data on antifibrotics use for RA-ILD in clinical practice (CP) are scarce. Our aim was: a) To assess the efficacy of antifibrotic drugs, NINTE and pirfenidone (PIRFE), in Spanish RA-ILD patients with a progressive phenotype in CP; b) To compare the profile of CP RA-ILD patients with those included in the INBUILD trial.
Methods: National multicenter study of RA-ILD patients to whom NINTE or PIRFE were added due to progressive fibrosing ILD. Demographic and clinical variables were collected from all patients. These features were compared with those of RA-ILD patients included in the INBUILD trial (n=89, 42 NINTE and 47 placebo). Forced vital capacity (FVC) evolution was the primary endpoint. Results are expressed as percentage, mean±SD or median [IQR].
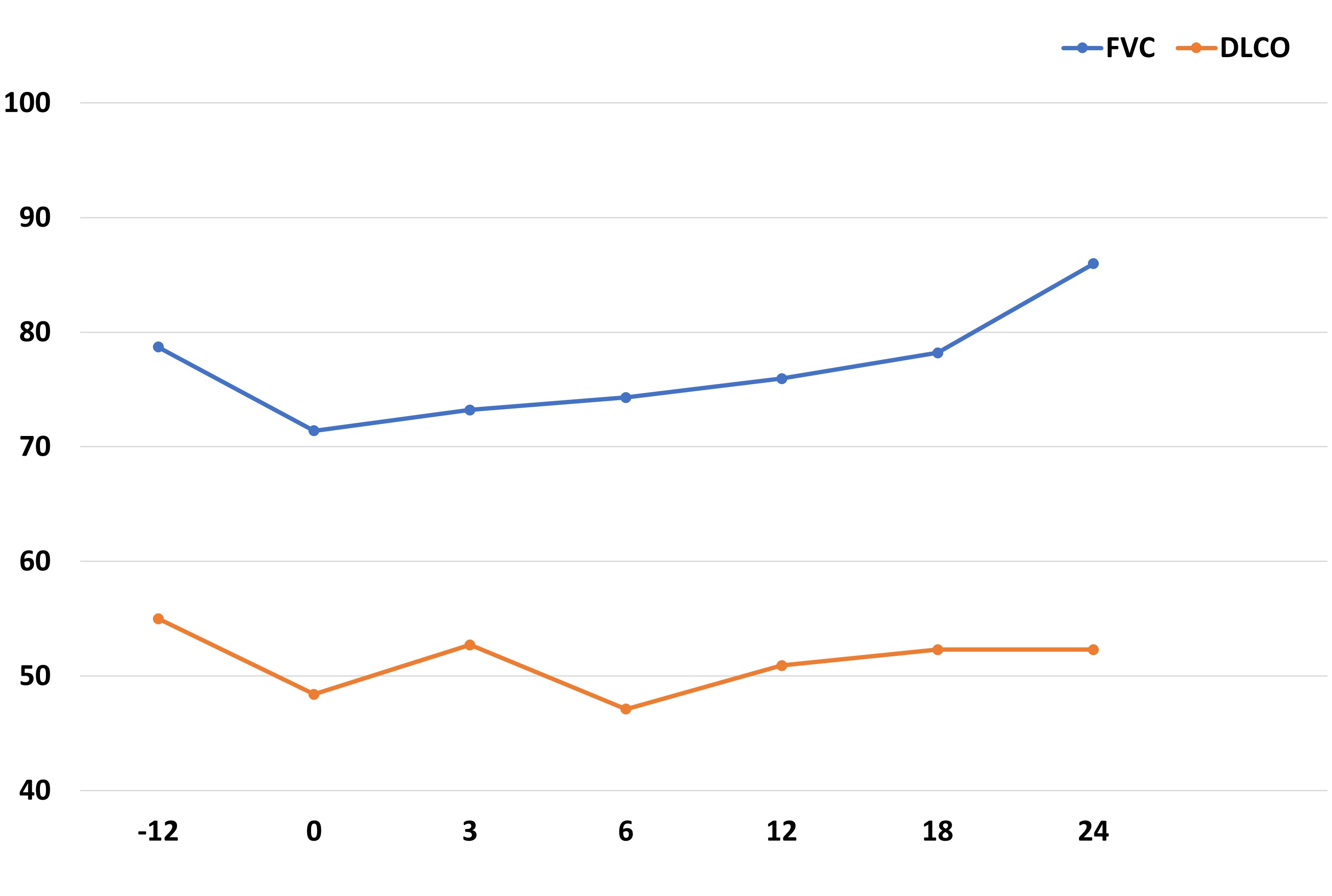
Results: A total of 73 patients (24 women/49 men) from CP were collected, mean age of 69±9 years. The median [IQR] ILD duration up to antifibrotic initiation was of 56.5 [21.5-83] months. NINTE was the most widely used antifibrotic (n=66), combined with immunosuppressive treatment in all cases: corticosteroids in 44, cDMARD in 20 (mycophenolate mofetil=8, leflunomide=6, methotrexate=3, hydroxychloroquine=2, azathioprine=1), bDMARD in 43 (abatacept=31, rituximab=10, anti-IL6R=2) and/or JAKi in 4 (baricitinib=2, tofacitinib=1, filgotinib=1). Mean FVC one year before NINTE start was 80±21 (% pred.), whilst mean baseline FVC was 71±23 (% pred.). Table 1 shows the comparison of baseline characteristics of RA-ILD patients treated with NINTE in CP and in the INBUILD trial. The evolution of FVC and diffusing capacity of the lungs for carbon monoxide (DLCO) in our patients with NINTE from the previous year of antifibrotic initiation is shown in Figure 1. After a median follow-up of 15.5 [3.5-23.5] months, no significant decline in mean FVC or DLCO values was observed. In addition, 86% of the patients presented stabilization or improvement of dyspnea. NINTE was withdrawn in 14 patients due to: gastrointestinal adverse events (11), death (1), hemorrhage risk (1), and stabilization (1). PIRFE was administered to 7 patients (4 men), combined with abatacept in 3 patients, leflunomide in 1, and methotrexate in 1. Mean baseline FVC and DLCO were 69±22 and 49±14 (% pred.), respectively. As with NINTE, a stability in the evolution of lung function was observed. PIRFE was withdrawn in 4 patients due to: gastrointestinal adverse events (2), inefficacy (1), and stabilization (1).
Conclusion: Antifibrotics, especially NINTE, seem to slow ILD progression in patients with RA-ILD. In CP, our patients are treated belatedly in the evolution of the disease, but results are satisfactory. Combination of antifibrotics and DMARDs is feasible and safe.
.jpg)
B. Atienza-Mateo: None; A. Serrano-Combarro: None; M. Martin-Lopez: None; S. Castañeda: None; R. Melero-Gonzalez: None; J. Loarce-Martos: Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Galapagos, 6; N. Mena Vazquez: None; C. carrasco-Cubero: None; C. Diez-Morrondo: None; D. Castro-Corredor: None; T. Vazquez-Rodriguez: None; a. Garcia-Valle: None; G. Bonilla: None; M. Rodriguez-lopez: None; I. Brana Abascal: None; S. ROJAS HERRERA: None; J. Sarmiento-Monroy: None; P. Andujar-Brazal: None; J. De Dios: None; C. Gonzalez-Montagut Gomez: None; S. Ordonez-Palau: None; A. Brandy-Garcia: None; F. Lozano: None; M. Lopez-Lasanta: None; C. Campos Fernández: None; M. Garijo Bufort: None; I. Casafont-Sole: None; C. Goercke: None; C. Iñiguez: None; F. Ortiz-Sanjuán: Eli Lilly, 6, Grunenthal, 2, GSK, 2; E. Giner-Serret: None; B. Flores Robles: None; M. Moreno: None; E. Cervantes-Perez: None; D. Ferrer: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; O. Collaborative Group Members: None.
Background/Purpose: Interstitial lung disease (ILD) is a critical complication of rheumatoid arthritis (RA). Abatacept and rituximab are the preferred disease-modifying antirheumatic drugs (DMARDs) for RA-ILD. However, progression of ILD despite its use is not uncommon. A subgroup analysis of the INBUILD trial has shown a slower decline in forced vital capacity (FVC) in patients with progressive fibrosing autoimmune disease-related ILD with nintedanib (NINTE) [Arthritis Rheumatol 2022;74(6):1039-47]. However, data on antifibrotics use for RA-ILD in clinical practice (CP) are scarce. Our aim was: a) To assess the efficacy of antifibrotic drugs, NINTE and pirfenidone (PIRFE), in Spanish RA-ILD patients with a progressive phenotype in CP; b) To compare the profile of CP RA-ILD patients with those included in the INBUILD trial.
Methods: National multicenter study of RA-ILD patients to whom NINTE or PIRFE were added due to progressive fibrosing ILD. Demographic and clinical variables were collected from all patients. These features were compared with those of RA-ILD patients included in the INBUILD trial (n=89, 42 NINTE and 47 placebo). Forced vital capacity (FVC) evolution was the primary endpoint. Results are expressed as percentage, mean±SD or median [IQR].
Results: A total of 73 patients (24 women/49 men) from CP were collected, mean age of 69±9 years. The median [IQR] ILD duration up to antifibrotic initiation was of 56.5 [21.5-83] months. NINTE was the most widely used antifibrotic (n=66), combined with immunosuppressive treatment in all cases: corticosteroids in 44, cDMARD in 20 (mycophenolate mofetil=8, leflunomide=6, methotrexate=3, hydroxychloroquine=2, azathioprine=1), bDMARD in 43 (abatacept=31, rituximab=10, anti-IL6R=2) and/or JAKi in 4 (baricitinib=2, tofacitinib=1, filgotinib=1). Mean FVC one year before NINTE start was 80±21 (% pred.), whilst mean baseline FVC was 71±23 (% pred.). Table 1 shows the comparison of baseline characteristics of RA-ILD patients treated with NINTE in CP and in the INBUILD trial. The evolution of FVC and diffusing capacity of the lungs for carbon monoxide (DLCO) in our patients with NINTE from the previous year of antifibrotic initiation is shown in Figure 1. After a median follow-up of 15.5 [3.5-23.5] months, no significant decline in mean FVC or DLCO values was observed. In addition, 86% of the patients presented stabilization or improvement of dyspnea. NINTE was withdrawn in 14 patients due to: gastrointestinal adverse events (11), death (1), hemorrhage risk (1), and stabilization (1). PIRFE was administered to 7 patients (4 men), combined with abatacept in 3 patients, leflunomide in 1, and methotrexate in 1. Mean baseline FVC and DLCO were 69±22 and 49±14 (% pred.), respectively. As with NINTE, a stability in the evolution of lung function was observed. PIRFE was withdrawn in 4 patients due to: gastrointestinal adverse events (2), inefficacy (1), and stabilization (1).
Conclusion: Antifibrotics, especially NINTE, seem to slow ILD progression in patients with RA-ILD. In CP, our patients are treated belatedly in the evolution of the disease, but results are satisfactory. Combination of antifibrotics and DMARDs is feasible and safe.
.jpg)
Table 1. Baseline characteristics of RA-ILD patients treated with NINTE in clinical practice and RA-ILD patients included in the INBUILD trial.

Figure 1. Evolution of FVC and DLCO in 66 patients with RA-ILD treated with nintedanib in clinical practice since the previous year of initiation.
B. Atienza-Mateo: None; A. Serrano-Combarro: None; M. Martin-Lopez: None; S. Castañeda: None; R. Melero-Gonzalez: None; J. Loarce-Martos: Boehringer-Ingelheim, 6, Bristol-Myers Squibb(BMS), 6, Galapagos, 6; N. Mena Vazquez: None; C. carrasco-Cubero: None; C. Diez-Morrondo: None; D. Castro-Corredor: None; T. Vazquez-Rodriguez: None; a. Garcia-Valle: None; G. Bonilla: None; M. Rodriguez-lopez: None; I. Brana Abascal: None; S. ROJAS HERRERA: None; J. Sarmiento-Monroy: None; P. Andujar-Brazal: None; J. De Dios: None; C. Gonzalez-Montagut Gomez: None; S. Ordonez-Palau: None; A. Brandy-Garcia: None; F. Lozano: None; M. Lopez-Lasanta: None; C. Campos Fernández: None; M. Garijo Bufort: None; I. Casafont-Sole: None; C. Goercke: None; C. Iñiguez: None; F. Ortiz-Sanjuán: Eli Lilly, 6, Grunenthal, 2, GSK, 2; E. Giner-Serret: None; B. Flores Robles: None; M. Moreno: None; E. Cervantes-Perez: None; D. Ferrer: None; R. Blanco: AbbVie, 5, 6, Amgen, 6, AstraZeneca, 2, BMS, 6, Eli Lilly, 6, Galapagos, 2, 6, Janssen, 2, 6, MSD, 6, Novartis, 2, 6, Pfizer, 2, 6, Roche, 5, 6, Sanofi, 6; O. Collaborative Group Members: None.



