Poster Session A
Crystal arthropathies
0246: Safety & Efficacy of SEL-212 in Patients with Gout Refractory to Conventional Treatment: Primary Outcomes from Two Randomized, Double Blind, Placebo-Controlled, Multicenter Phase 3 Studies

Herbert Baraf, MD, FACP, MACR
National Institute of Arthritis and Musculoskeletal and Skin Diseases
Bethesda, MD, United StatesDisclosure(s): Horizon Pharmaceuticals: Grant/Research Support (Ongoing), Speaker/Honoraria (includes speakers bureau, symposia, and expert witness) (Ongoing); Swedish Orphan Biovitrum (Sobi): Grant/Research Support (Ongoing), Speaker/Honoraria (includes speakers bureau, symposia, and expert witness) (Ongoing)
Abstract Poster Presenter(s)
Background/Purpose: In patients with refractory gout, the inability to maintain serum uric acid (sUA) levels < 6 mg/dL leads to severe clinical manifestations for which uricase-based therapies can be highly effective, though also immunogenic.1 SEL-212 is a once-monthly, novel 2-component, uricase-based infusion therapy being investigated in patients with refractory gout. SEL-212 consists of an infusion of tolerogenic nanoparticles containing rapamycin (SEL-110) followed by pegadricase (SEL-037).2 DISSOLVE I and II (D1 and D2, respectively) evaluated the safety and efficacy of SEL-212 in adults with refractory gout.
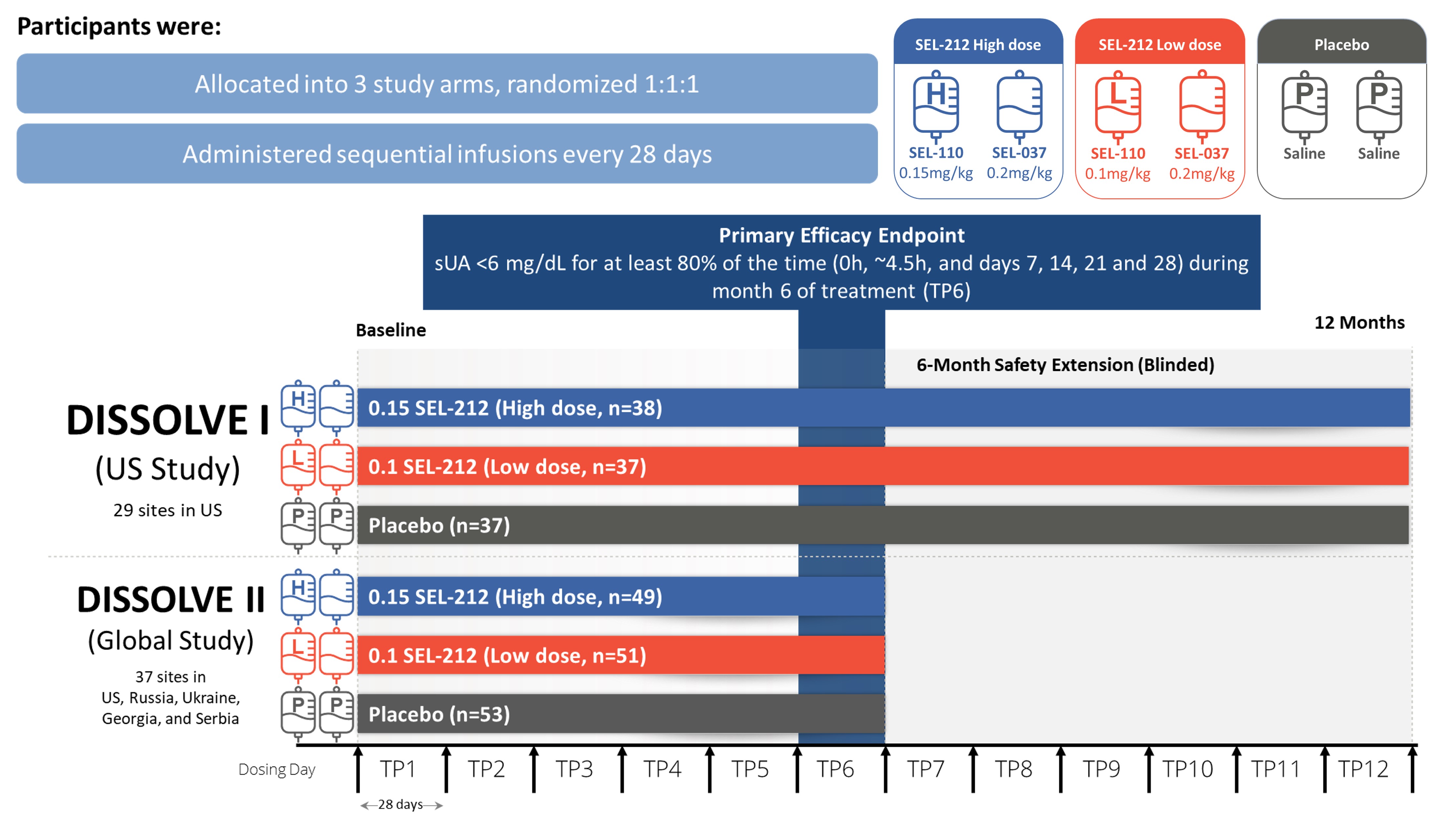
Methods: D1 (US) and D2 (Global), were placebo-controlled, double-blind, randomized clinical trials that evaluated two dose levels of SEL-110 (0.15 mg/kg [high-dose] or 0.1 mg/kg [low-dose]) prior to SEL-037 (0.2 mg/kg) infusion. Participants were enrolled if they had ≥ 3 gout flares within 18 months prior to screening or ≥ 1 tophus or a current diagnosis of gouty arthritis, failed to normalize sUA and control symptoms with any xanthine oxidase inhibitor, and were not previously exposed to a pegylated uricase-based therapy. Participants were randomized 1:1:1 between the two doses of SEL-212 and placebo administered intravenously every 28 days for 6 treatments. D1 participants continued in a 6-month blinded extension phase under the initial treatment conditions (Fig. 1). The primary endpoint was defined as the percentage of participants who achieved and maintained sUA < 6 mg/dL for ≥ 80% of the sixth treatment period (TP6). Safety and tolerability were assessed through monitoring of adverse events (AEs).
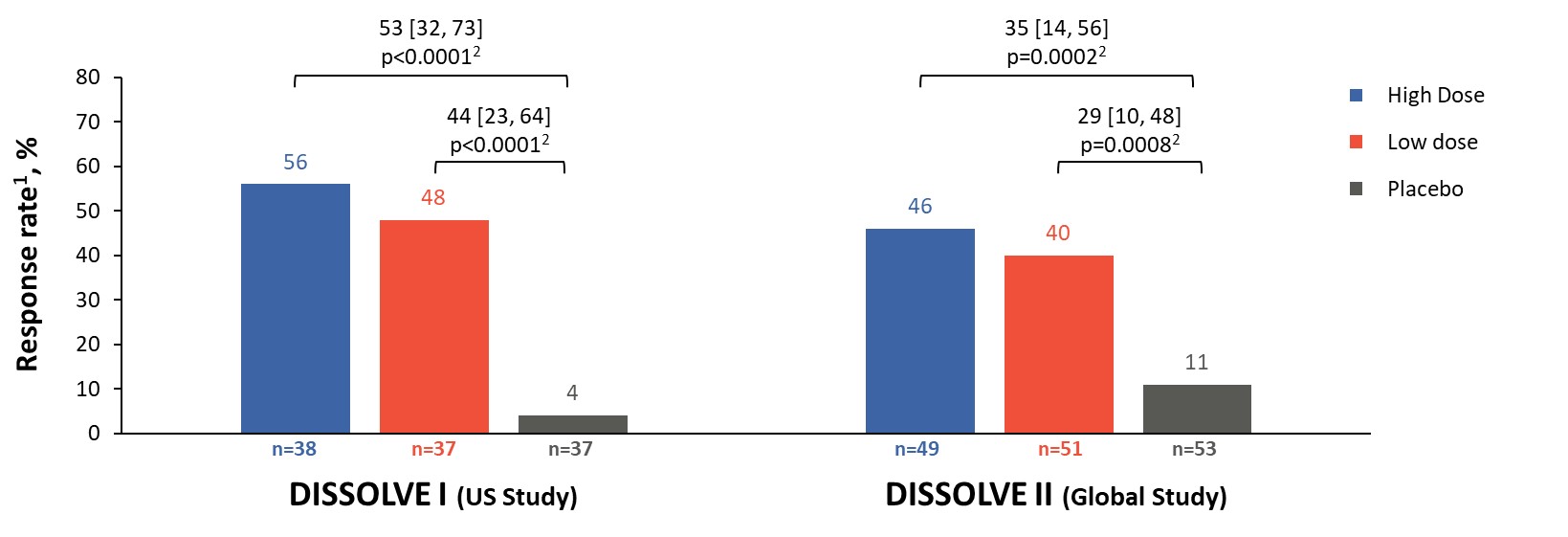
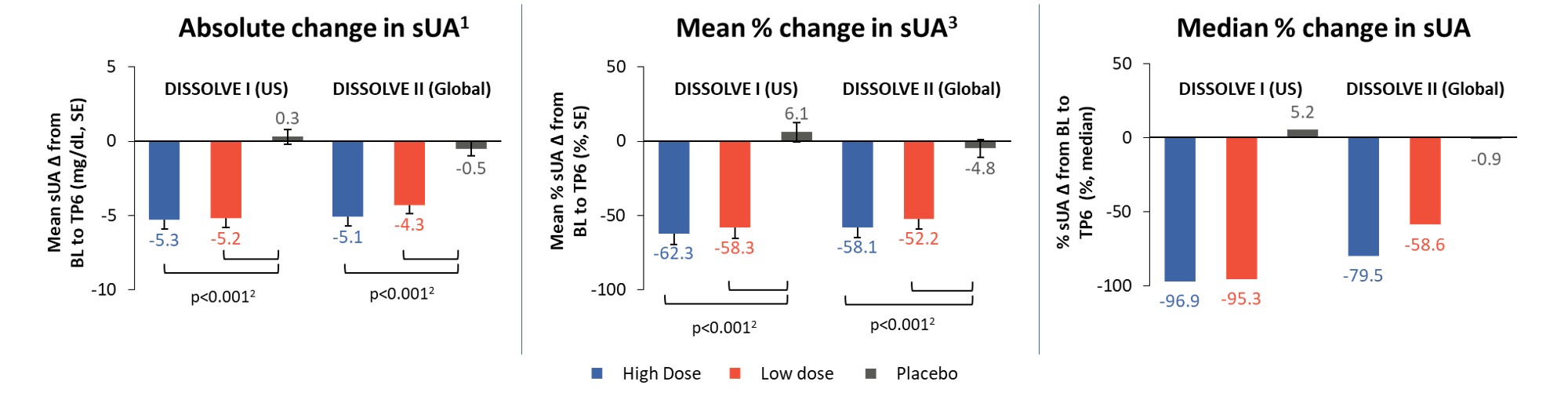
Results: 112 participants (96% male, 66% ≥ 50 years) were enrolled in D1 and 153 (97% male, 72% ≥ 50 years) in D2. Response rates in all treatment groups were significantly different from placebo (p ≤ 0.0008), with 56% and 46% of participants responding in the high-dose group and 48% and 40% in the low-dose group for D1 and D2, respectively (Figure 2). The response rates in participants aged ≥ 50 years were 65% and 47% in the high-dose groups and 47% and 44% in the low-dose groups for D1 and D2, respectively (p ≤ 0.0026 vs placebo). Across all participants in the treatment groups, sUA levels were significantly reduced from baseline (p< 0.001 vs placebo) at TP6 (Figure 3). The safety profile of SEL-212 was favorable, with 3.4% and 4.5% of participants experiencing infusion reactions in the high and low-dose groups, respectively. Reports of gout flares were comparable between treatment groups and placebo. Six participants (3.4%) in the pooled active treatment groups experienced treatment-related serious AEs (n=4 anaphylaxis, n=2 gout flares).
Conclusion: In the DISSOLVE trials, once-monthly treatment with SEL-212 demonstrated statistically significant response rates and reductions in sUA versus placebo. The safety profile of SEL-212 was consistent with that of uricase therapies. Targeted immunomodulation with SEL-212 has the potential to provide a new uricase-based treatment option for patients with gout refractory to conventional therapies.
- Edwards NL. Arthritis Rheum 2008;58(9):2587-90.
- Sands E, et al. Nat Commun 2022;13:272.
H. Baraf: Horizon Pharmaceuticals, 5, 6, Swedish Orphan Biovitrum (Sobi), 5, 6; A. Kivitz: AbbVie, 6, Amgen, 6, 11, Chemocentryx, 1, Eli Lilly, 6, Fresenius Kabi, 2, Genzyme, 2, Gilead, 2, 11, GlaxoSmithKlein (GSK), 2, 6, 11, Grunenthal, 2, Horizon, 1, 2, Janssen, 1, 2, Novartis, 4, 11, Pfizer, 2, 6, 11, Selecta, 2, Synact, 2, Takeda, 2, UCB, 1, 6; S. Rhodes: Selecta Biosciences, 3, 11; S. Leung: Selecta Biosciences, 3, 11; O. Folarin: Selecta Biosciences, 3, 11; T. Gonzalez-Rivera: Swedish Orphan Biovitrum (Sobi), 3; J. Sobierska: Swedish Orphan Biovitrum (Sobi), 3; J. Christie: GlaxoSmithKlein(GSK), 11, Swedish Orphan Biovitrum (Sobi), 3; A. Patel: Lexicon Pharmaceuticals, 6; W. DeHaan: Selecta Biosciences, 3, 11; R. Azeem: Selecta Biosciences, 3, 11; P. Traber: Selecta Biosciences, 3, 11.



